Difference between revisions of "Part:BBa K404122"
| Line 483: | Line 483: | ||
was plotted against increasing ganciclovir concentrations. </span></p> | was plotted against increasing ganciclovir concentrations. </span></p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<p class=MsoNormal><span lang=EN-US> </span></p> | <p class=MsoNormal><span lang=EN-US> </span></p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<h3><a name="_Toc275981848"><span lang=EN-US>Conclusions</span></a></h3> | <h3><a name="_Toc275981848"><span lang=EN-US>Conclusions</span></a></h3> | ||
| Line 627: | Line 496: | ||
offering a feasible and modular tool to the growing field of personalized | offering a feasible and modular tool to the growing field of personalized | ||
medicine and the iGEM community. We successfully demonstrated cancer cell death | medicine and the iGEM community. We successfully demonstrated cancer cell death | ||
| − | caused | + | caused the modified fusion gene |
consisting of guanylate and thymidine kinases.</span></p> | consisting of guanylate and thymidine kinases.</span></p> | ||
| Line 634: | Line 503: | ||
efficiently retargeted the viral vector for directed suicide gene delivery | efficiently retargeted the viral vector for directed suicide gene delivery | ||
towards tumor cells. Capsid engineering was successfully demonstrated by the | towards tumor cells. Capsid engineering was successfully demonstrated by the | ||
| − | iGEM team Freiburg_Bioware 2010. Further details can be found | + | iGEM team Freiburg_Bioware 2010. Further details can be found on the wiki of the Freiburg_Bioware 2010 |
| − | Targeting.</span></p> | + | <a href="http://2010.igem.org/Team:Freiburg_Bioware/Project/Results#targeting">Results – |
| + | Targeting.</a></span></p> | ||
<h3><a name="_Toc275981849"><span lang=EN-US>References</span></a></h3> | <h3><a name="_Toc275981849"><span lang=EN-US>References</span></a></h3> | ||
Revision as of 20:54, 27 October 2010
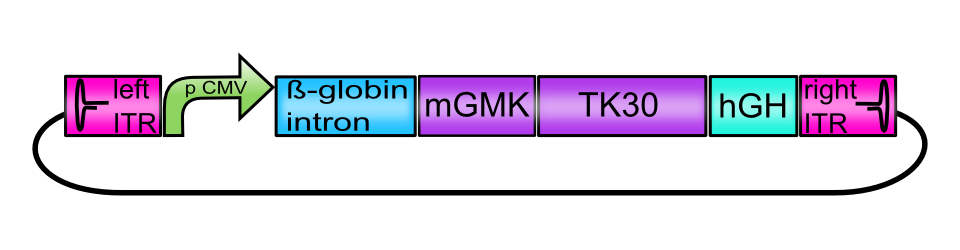
[AAV2]-left-ITR_pCMV_betaglobin_mGMK_TK30_hGH_[AAV2]-right-ITR
This composite BioBrick serves as an example for the possibilities of our Virus Construction Kit. It has been assembled out of 7 single BioBricks: A promoter, a transcription enhancing sequence, a Gene of Interest that is itself a fusion protein, a polyadenylation sequence required for translation and inverted terminal repeats that are required for replication at either end of the sequence. If cotransfected into helper cell lines together with a plasmid encoding AAV Rep and Cap proteins and a pHelper plasmid encoding adenoviral genes, this BioBrick is being packaged into AAV capsids in single-stranded form and substitutes the AAV genome. AAV particles containing this Biobrick as a vector sequence are replication-deficient and are therefore considered as biosafety level 1.
Contents
Successful Assembly of Vector Plasmids Carrying Suicide Genes via Cloning
Monitoring Efficient Tumor Killing by Phase-Contrast Microscopy
Quantitative Analysis of Cell Death by Flow Cytometry
Titrating Ganciclovir Concentrations for Efficient Cell Killing by Cytotoxicity Assays
Killing Untransduced Tumor Cells via Bystander Effect
Arming: Suicide Genes as GOIs
Introduction
Gene delivery using viral vectors to specifically target tumor cells gained increasing attention in the last years being efficient in combination with suicide gene approaches (Willmon et al. 2006). Several prodrug/enzyme combinations have been reported. The two systems - ganciclovir (GCV)/herpes simplex virus thymidine kinase (HSV-TK) (Ardiani et al. 2010) and 5-fluorocytosine/cytosine deaminase (CD) (Fuchita et al. 2009a) – have been widely used and their therapeutic benefit was demonstrated in preclinical studies (Greco & Dachs 2001). Adeno-associated viruses (AAV) as delivery vectors are commonly used in suicide gene therapy. The suicide gene flanked by the inverted terminal repeats (ITRs) is encapsulated into the virus particles and delivered to the target cells where suicide gene expression is mediated by cellular proteins.
The iGEM team Freiburg_Bioware 2010 provides both the cytosine deaminase (CD, BBa_K404112) and an improved guanylate kinase - thymidine kinase fusion gene (mGMK_TK, BBa_K404113) within the Virus Construction Kit as effective suicide genes. We demonstrate efficient and specific killing of tumor cells by enzymatic cytotoxicity assays, flow cytometry, as well as phase contrast microscopy. HT1080 cancer cell lines were transduced with directed viral particles containing the suicide genes packaged into the viral capsids.
Successful Assembly of Vector Plasmids Carrying Suicide Genes via Cloning
Assembly of the constructs carrying the suicide genes (termed vector plasmids) was performed following the BioBrick Standard Assembly. All plasmids contain the enhancer-element human beta-globin intron (BBa_K404107) and the human growth hormone terminator signal (hGH, BBa_K404108) flanked by the inverted terminal repeats (ITRs, BBa_K404100 and BBa_K404101). Assembled suicide genes are either under the control of the CMV promoter or the tumor-specific telomerase promoter phTERT (BBa_K404106).
|
Figure 1: BioBrick compatible assembly of functional vector plasmids containing the suicide genes. The schematic figure shows the cloning strategy of the guanylate kinase – thymidine kinase fusion gene (mGMK_TK30). |
In order to modularize thymidine kinase mutants TK30 and SR39 (BBa_K404109 and BBa_K404110) according to the BioBrick standard, the fusion genes mGMK_TK30 and mGMK_SR39 (BBa_K404113 and BBa_K404315) and CD (BBa_K404112), were modified using the QuikChange Lightning Site-Directed Mutagenesis Kit (Stratagene) for deletion of iGEM pre- and suffix restriction sites. Figure 1 demonstrates one example of successful deletion of the PstI restriction site located within the mGMK_TK30 sequence at position 3109. Base pair exchange was introduced by replacing the nucleotide G with A, resulting in the deletion of the restriction site, but maintaining the amino acid glutamine. Successful transition of G to A was confirmed by sequencing (Figure 2).
|
Figure 2: Replacing the nucleotide G with C by site-directed mutagenesis using QuikChange Lightning Kit provided by Stratagene has been successful performed as demonstrated by (A) test digestion linearizing the plasmid with PstI and (B) by sequencing. |
Furthermore, assembly of BioBrick-compatible vector plasmids was performed. An example for the last assembly step of mGMK_TK30 and hGH_rITR is shown in Figure 3. The plasmids were digested with both XbaI and PstI (Insert: BBa_K404116: hGH_rITR) or SpeI and PstI (Vector) and loaded on an agarose gel. As demonstrated in the preparative gel in Figure 3, the expected bands were detected under UV light and the extracted DNA was be successfully ligated. Each assembly step for producing BioBricks was conducted following the iGEM BioBrick standard.
|
Figure 3: Cloning of the composite parts mGMK_TK30 to hGH-terminator_rightITR (insert). The digested fragments correspond to the expected sizes. |
Monitoring Efficient Tumor Killing by Phase-Contrast Microscopy
Tumor cells, transduced with viral particles encapsidating the effector constructs containing the mGMK_TK30 driven by the CMV promoter, were cultured in presence and absence of ganciclovir. Morphological changes were monitored via phase-contrast microscopy until 48 hours post infection. As it can be seen in Figure 4 non-transduced cells treated with ganciclovir and transduced cell without ganciclovir did not show significant tumor cell ablation. In contrast transduced cells expressing the guanylate kinase - thymidine kinase fusion protein, showed significant cell death after incubation with ganciclovir for 48 hours post infection.
|
A |
B |
|
C
|
D |
|
Figure 4: Qualitative analysis of cell death induced by conversion of ganciclovir to ganciclovir-triphosphate by virus-delivered guanylate - thymidine kinase (mGMK_TK30). A: Non-transduced HT1080 cells incubated in the presence of ganciclovir did not exhibit cell death. B: Transduced HT1080 cells untreated resulting in survival of most cells. C: HT1080 cells were transduced with 300µL viral particles and incubated with ganciclovir leading to ablation of tumor cells. D: HT1080 cells were transduced with 600µL viral particles and incubated with ganciclovir leading to ablation of tumor cells. |
|
Suicide gene therapy is based on the conversion of non-toxic prodrugs to toxic substances (Greco & Dachs 2001), leading to cell death (Figure 5). Directed gene delivery is achieved by using recombinant viral vectors as provided by the iGEM team Freiburg_Bioware 2010 within the Virus Construction Kit.
|
Figure 5: Overview over the suicide gene therapy approach. Non-toxic prodrugs are converted into toxic effector molecules leading to cell death of the tumor cells. |
Since ganciclovir is not toxic for cells, non-transduced cells can survive in the presence of the prodrug (Figure 4A). Demonstrating that transduced cells are viable in absence of ganciclovir, confirms that cell killing is induced by combination of delivered thymidine kinase and treatment with ganciclovir. Viral particles encapsidating the suicide construct mGMK_TK30 are efficient in directed gene delivery, thus leading to cell death of transduced cells due to overexpression of mGMK_TK30 and prodrug conversion. The cell toxic ganciclovir-triphosphate is incorporated into the nascent DNA chain leading to replication termination and finally resulting in death of dividing cells.
Quantitative Analysis of Cell Death by Flow Cytometry
Quantitative analysis of the cytotoxic effect induced by mGMK_TK30 was first conducted by flow cytometry analysis 72 hours post transduction. HT1080 cells were stained with 7-AAD and Annexin V. 7-AAD intercalates in double-stranded DNA after penetrating cell membranes of dead cells, whereas Annexin V binds specifically phosphatidylserine which is only accessible during apoptosis. Figure 6 demonstrates the relation between cell death and ganciclovir concentration.
|
|
|
|
Figure 6: A: Gating non-transduced HT1080 cells (control). B: Non-transduced cells without staining plotted against 7-AAD. C: Gating non-transduced cells stained with 7-AAD. D: Non-transduced, 7-AAD-stained cells plotted against 7-AAD. E: Gating transduced cells (GOI: mGMK_TK30) treated with 485µM Ganciclovir. F: Gated, Annexin-V stained cells plotted against AnnV-2 Log. G: Gated cells plotted against 7-AAD H: Gated, 7-AAD and Annexin-V stained cells plotted against 7-AAD and Annexin-V. Gate R19 comprised Annexin-V and 7-AAD positive cells. |
|
|
Figure 7: Quantification of flow cytometry data provided in Figure 6. With increasing ganciclovir concentration, the survival rate of cells decreases. 60% of HT1080 cells treated with 4,85 mM ganciclovir show tumor ablation, however even lower amounts of ganciclovir led to significant cell death. |
Effect of different ganciclovir concentrations on transduced HT1080 sarcoma cells. Transduction has been performed with recombinant viral particles encapsidating the mGMK_TK30 prodrug gene. 72 hours post infection cells were stained with 7-AAD and Annexin V. As Figure 7 shows, the higher the ganciclovir concentration, the more transduced cells were killed.
Titrating Ganciclovir Concentrations for Efficient Cell Killing by Cytotoxicity Assays
Further analysis of the cytotoxic effect induced by thymidine kinase converting ganciclovir to the toxic anti-metabolite has been performed using MTT assays. 3-(4,5-Dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide), also known as MTT, is a yellow tetrazole, which is reduced to purple insoluble formazan in the presence of NADH and NADPH (Roche n.d.). The colorimetric analysis can be carried out via spectrometry. Different tumor cell lines, HT1080 and A431, were transduced with the recombinant viruses carrying the linear DNA construct coding for mGMK-TK30 regulated by the CMV promoter and treated with ganciclovir. 48 and 72 hours post infection cells were incubated with MTT and absorbance of formazan was quantified.
|
A |
|
B
Figure 8: Effect of ganciclovir on HT1080 cell killing 72 hours post infection as (A) two dimensional plot of survival of cells and (B) three-dimensional plot of ganciclovir, virus particles and cell survival. |
|
A
|
|
B
Figure 9: Effect of ganciclovir on HT1080 cell killing 96 hours post infection as (A) two dimensional plot of survival of cells and (B) three-dimensional plot of ganciclovir, virus particles and cell survival. |
Data of MTT assay quantification are shown in Figure 8 and Figure 9. HT1080 cells were infected with viral particles containing the mGMK_TK30 transgene. 72 h- and 96 h post infection and addition of ganciclovir, cells were incubated with MTT. Changes in absorbance were measured and survival of cells plotted against ganciclovir concentration. Figure 8A demonstrates the correlation between increasing ganciclovir concentrations and percentage of cell survival. Furthermore, different virus particle concentrations were used for transduction. Figure 8B shows that the highest amount of viral particles combined with the highest ganciclovir concentration led to significant HT1080 apoptosis 72 hours post transduction.
Additionally 96 hours post infection cells were incubated with MTT and absorbance was quantified via spectrometry (Figure 9). Again, survival of HT1080 cells was plotted against increasing ganciclovir concentrations.
Conclusions
Efficient and tissue-specific tumor killing is one major challenge in cancer therapy (Black et al. 1996). Gene-directed enzyme prodrug therapy (GDEPT) is based on the conversion of non-toxic substances into toxic drugs resulting in tumor cell death. The iGEM team Freiburg_Bioware 2010 provides several functional suicide genes within the Virus Construction Kit. Thus offering a feasible and modular tool to the growing field of personalized medicine and the iGEM community. We successfully demonstrated cancer cell death caused the modified fusion gene consisting of guanylate and thymidine kinases.
To prevent systemic toxic side effects of conventional chemotherapy the iGEM team Freiburg_Bioware 2010 took a leap and efficiently retargeted the viral vector for directed suicide gene delivery towards tumor cells. Capsid engineering was successfully demonstrated by the iGEM team Freiburg_Bioware 2010. Further details can be found on the wiki of the Freiburg_Bioware 2010 Results – Targeting.
References
Ardiani, A., Sanchez-Bonilla, M. & Black, M.E., 2010. Fusion enzymes containing HSV-1 thymidine kinase mutants and guanylate kinase enhance prodrug sensitivity in vitro and in vivo. Cancer gene therapy, 17(2), 86-96. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2808426&tool=pmcentrez&rendertype=abstract.
Black, M.E. et al., 1996. Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy. Proceedings of the National Academy of Sciences of the United States of America, 93(8), 3525-9. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=39643&tool=pmcentrez&rendertype=abstract.
Fuchita, M. et al., 2009. Bacterial cytosine deaminase mutants created by molecular engineering show improved 5-fluorocytosine-mediated cell killing in vitro and in vivo. Cancer research, 69(11), 4791-9. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2765227&tool=pmcentrez&rendertype=abstract.
Fuchita, M. et al., 2009. Bacterial cytosine deaminase mutants created by molecular engineering show improved 5-fluorocytosine-mediated cell killing in vitro and in vivo. Cancer research, 69(11), 4791-9. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2765227&tool=pmcentrez&rendertype=abstract.
Greco, O. & Dachs, G.U., 2001. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. Journal of cellular physiology, 187(1), 22-36. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11241346.
Huber, B.E. et al., 1993. In vivo antitumor activity of 5-fluorocytosine on human colorectal carcinoma cells genetically modified to express cytosine deaminase. Cancer research, 53(19), 4619-26. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8402637.
Huber, B.E. et al., 1994. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proceedings of the National Academy of Sciences of the United States of America, 91(17), 8302-6. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=44594&tool=pmcentrez&rendertype=abstract.
Moolten, F.L., 1986. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer research, 46(10), 5276-81. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3019523.
Roche, Apoptosis , Cell Death and Cell Proliferation,
Willmon, C.L., Krabbenhoft, E. & Black, M.E., 2006. A guanylate kinase/HSV-1 thymidine kinase fusion protein enhances prodrug-mediated cell killing. Gene therapy, 13(17), 1309-12. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16810197.
ITRs

The inverted terminal repeat structures can be subdivided into several palindromic motives: A and A’ form a stem loop which encases B and B’ as well as C and C’. Those motives form both arms of the T-shaped structure. The functional motives on the ITR are two regions that bind Rep 68/78, called Rep-binding elements (RBE on the stem and RBE’ on the B arm) and the terminal resolution site (trs) in which the rep proteins introduce single-stranded nicks. The 3’ OH end of the A motive acts as a primer for DNA replication (Im & Muzyczka, 1990) (Lusby, Fife, & Berns, 1980).
CMV promoter
Beta-globin-intron


The beta-globin intron BioBrick consists of a partial chimeric CMV promoter followed by the intron II of the beta-globin gene. The 3´end of the intron is fused to the first 25 bases of human beta globin gene exon 3. The beta globin intron BioBrick is assumed to enhance eukaryotic gene expression (Nott, Meislin, & Moore, 2003). As shown in Figure 9 and Figure 10 the vectorplasmid missing the beta-globin intron showed a negligible difference in mVenus expression compared to viral genomes containing the beta-globin intron. Considering these results and taking into account that a constant volume of viral particles has been used for transduction, the difference between the construct containing and lacking the beta-globin intron is minimal. Since packaging efficiency of the AAV-2 decreases with increasing sizes of the insert (Dong, Fan, & Frizzell, 1996), the iGEM team Freiburg_Bioware suggests using the beta-globin intron in dependence on the size of your transgene.
mGMK_TK30 fusion protein
The thymidine kinase mutant TK30 contains six modified amino acids (Black, Newcomb, Wilson, & Loeb, 1996) created in a first screening showing enhanced affinity for gancivlocir and acyclovir, but reduced specificity for its natural substrate thymidine.
As efficient tumor killing and therefore ganciclovir activation is essential for successful tumor ablation, further improvements were conducted. Overexpression of transgenic thymidine kinase leads to accumulation of non-toxic intermediates, which cannot be phosphorylated sufficiently by endogenous guanylate kinase, the second enzyme in the salvage pathway of nucleotides.
Overcoming this bottleneck was accomplished by fusing the mouse guanylate kinase (mGMK) to the N-terminus of TK30 mutant creating a fusion protein (mGMK_TK30) with enhanced GCV/ACV sensitivity in vitro and in vivo (Ardiani, Sanchez-Bonilla, & Black, 2010) and improved bystander activity. The effect of non-transfected tumor cell killing upon transfer of toxic metabolites through gap junctional intercellular communication (GJIC) or immune-mediated tumor ablation is essential in suicide gene therapy (Pope, 1997). GCV-triphosphate is mainly transported through the central pore formed between connexin proteins from neighboring cells (Gentry, Im, Boucher, Ruch, & Shewach, 2005), but immune-induced bystander effect seems to be likely as well (Grignet-Debrus, Cool, Baudson, Velu, & Calberg-Bacq, 2000).
hGH
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1319
Illegal NgoMIV site found at 1977
Illegal NgoMIV site found at 2882
Illegal AgeI site found at 3011 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 3413
Illegal SapI.rc site found at 1369