Difference between revisions of "Part:BBa K404224"
| Line 13: | Line 13: | ||
</div> | </div> | ||
| − | <div style="float:right; width: | + | <div style="float:right; width:400px; height:auto; "><img src="https://static.igem.org/mediawiki/parts/9/98/Freiburg10_ViralBrick_motif_Z34C.png" width="400" /></div></div> |
| − | height="auto"/></ | + | |
| − | </ | + | <br/><br/><br/><br/> |
| + | <h3>Capsid</h3> | ||
| + | The AAV capsid consists of 60 capsid protein subunits. The three cap proteins VP1, VP2, and VP3 are encoded in an overlapping reading frame. Arranged in a stoichiometric ratio of 1:1:10, they form an icosahedral symmetry. The mRNA encoding for the cap proteins is transcribed from p40 and alternative spliced to minor and major products. Alternative splicing and translation initiation of VP2 at a nonconventional ACG initiation codon promote the expression of VP1, VP2 and VP3. The VP proteins share a common C terminus and stop codon, but begin with a different start codon. The N termini of VP1 and VP2 play important roles in infection and contain motifs that are highly homologous to the phospholipase A2 (PLA2) domain and nuclear localization signals (BR)(+). | ||
| + | |||
| + | <h3>References</h3> | ||
| + | <b>DiPrimio, Asokan, Govindasamy, Agbandje-McKenna, & Samulski</b>, June 2008. Surface loop dynamics in adeno-associated virus capsid assembly. Journal of virology, 167(1), 5178–5189 <br /> | ||
| + | <center><img src="https://static.igem.org/mediawiki/parts/a/a7/Freiburg10_Cap_proteins_VP1_2%263.png" width="600" | ||
| + | height="auto"/></center></html><br/><br/><br/><br/> | ||
Revision as of 23:20, 27 October 2010
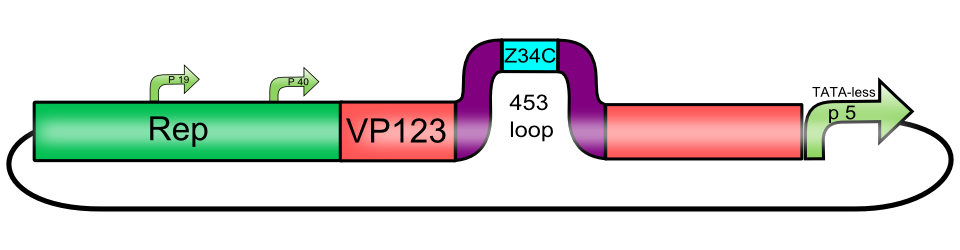
[AAV2]-Rep-VP123_P5-TATAless (ViralBrick-453-Z34C)
The Z34C antibody binding motif, inserted into the 453 loop of [AAV2]-Rep-VP123_p5-TATAless
Usage and Biology
The Z34C antibody binding motif is a 34 amino acids long fragment from the a minimized fragment of the Z Domain of Staphylococcal Protein A. It specifically binds to the Fc-Domain of antibodies. If inserted into AAV capsids, it can bind antibodies against a specific antigen, allowing differential targeting without the need for further genetic engineering.

Capsid
The AAV capsid consists of 60 capsid protein subunits. The three cap proteins VP1, VP2, and VP3 are encoded in an overlapping reading frame. Arranged in a stoichiometric ratio of 1:1:10, they form an icosahedral symmetry. The mRNA encoding for the cap proteins is transcribed from p40 and alternative spliced to minor and major products. Alternative splicing and translation initiation of VP2 at a nonconventional ACG initiation codon promote the expression of VP1, VP2 and VP3. The VP proteins share a common C terminus and stop codon, but begin with a different start codon. The N termini of VP1 and VP2 play important roles in infection and contain motifs that are highly homologous to the phospholipase A2 (PLA2) domain and nuclear localization signals (BR)(+).References
DiPrimio, Asokan, Govindasamy, Agbandje-McKenna, & Samulski, June 2008. Surface loop dynamics in adeno-associated virus capsid assembly. Journal of virology, 167(1), 5178–5189
Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 3713
Illegal XhoI site found at 1913
Illegal XhoI site found at 2099 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 4239
Illegal BsaI site found at 4421
Illegal BsaI site found at 4458
Illegal SapI site found at 3048