Difference between revisions of "Part:BBa K404210"
(→Usage and Biology) |
|||
| Line 12: | Line 12: | ||
The positively charged arginine residues interact with the HSPGs' negatively charged acid residues. Opie et al. have shown that two point mutations (R585A and R588A) are sufficient to eliminate the heparin binding affinity in AAV2. | The positively charged arginine residues interact with the HSPGs' negatively charged acid residues. Opie et al. have shown that two point mutations (R585A and R588A) are sufficient to eliminate the heparin binding affinity in AAV2. | ||
(Opie et al. 2003). This ViralBrick has been created to introduce this knockout into other constructs. The biobricks with containing this knockout are annotated with „HSPG-ko“. | (Opie et al. 2003). This ViralBrick has been created to introduce this knockout into other constructs. The biobricks with containing this knockout are annotated with „HSPG-ko“. | ||
| − | + | </div> | |
<div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/2010/0/04/Freiburg10_HSPG_binding_motif.png" width="460" | <div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/2010/0/04/Freiburg10_HSPG_binding_motif.png" width="460" | ||
| − | height="auto"/></div> | + | height="auto"/></div></div> |
| + | </html> | ||
Revision as of 18:55, 26 October 2010
ViralBrick-587KO-Empty
Usage and Biology
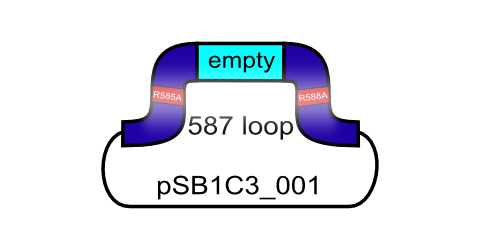
The primary receptor of AAV-2 is the heparan sulfate proteoglycan (HSPG) receptor (Perabo et al. 2006). Its binding motif consists of five amino-acids located on the capsid surface: R484/R487, K532, R585/587. (Trepel et al. 2009).
The positively charged arginine residues interact with the HSPGs' negatively charged acid residues. Opie et al. have shown that two point mutations (R585A and R588A) are sufficient to eliminate the heparin binding affinity in AAV2.
(Opie et al. 2003). This ViralBrick has been created to introduce this knockout into other constructs. The biobricks with containing this knockout are annotated with „HSPG-ko“.

===Restriction sites]]
Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 10
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]