Difference between revisions of "Part:BBa K404206"
(→Usage and Biology) |
(→Usage and Biology) |
||
| Line 6: | Line 6: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | |||
<div style="width:965px; height:400px; "> | <div style="width:965px; height:400px; "> | ||
<div style="float:left; width:460px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;"> | <div style="float:left; width:460px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;"> | ||
| Line 18: | Line 17: | ||
<div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/parts/5/59/Freiburg10_ViralBrick_motif_His.png" width="460" | <div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/parts/5/59/Freiburg10_ViralBrick_motif_His.png" width="460" | ||
height="auto"/></div></div><br><br><br><br> | height="auto"/></div></div><br><br><br><br> | ||
| − | |||
| − | |||
===Restriction sites=== | ===Restriction sites=== | ||
Revision as of 16:44, 26 October 2010
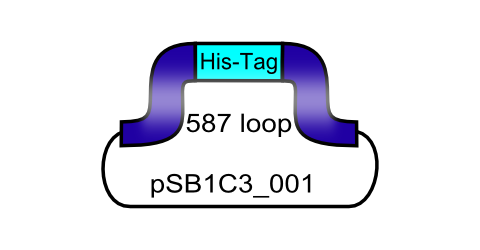
ViralBrick-587-His-Tag
Usage and Biology
Protein tagging via Histidine Tags is a widely used method for protein purification: Multiple histidine residues (most commonly: Six) are being fused tot he end of the targeting protein.
The high binding affinity of Histidine towards metal is being exploited for the purification of proteins via the so called „Immobilized Metal Ion Affinity Chromatography“ (IMAC): Multiple histidine residues (most commonly: Six) are being fused to the end of the targeting protein. A cell extract containing the recombinant protein ist then applied to a collumn containing immobilized Ni2+-Ions. The His-tags covalently bind the Ni-Ions while other cellular proteins can be washed oft he collumn. The purified proteins can then be eluted with Imidazol, which displaces the histidine residues.(Smith et al. 1988), (Hoffmann & Roeder 1991)
Since the aim behind engineering therapeutic AAV vectors is a safe administration to human patients, it is important to consider a convenient way of purifying the virus particles. Contamination by cellular proteins could cause toxic side effects or a strong immune response. Koerber et al. have first inserted a His-tag into a surface-exposed loop at amino acid position 587 in the Cap protein and successfully purified recombinant virsuses using IMAC (Koerber et al. 2007). For our Virus Construction Kit, we provide the His-tag motif in the ViralBrick standard, allowing for an easy insertion into the 453 and/or 587 loop. If the modified capsid bearing a His-tag is being cotransfected with a wild type capsid for the production of mosaic viruses, IMAC helps to not only purify the produced viral particles but also to enrich particles which actually contain the modified proteins.
References:
 " width="460"
height="auto"/>
" width="460"
height="auto"/>Restriction sites
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 10
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]