Difference between revisions of "Part:BBa K346003"
JjunyiJiao (Talk | contribs) |
JjunyiJiao (Talk | contribs) |
||
| Line 2: | Line 2: | ||
<partinfo>BBa_K346003 short</partinfo> | <partinfo>BBa_K346003 short</partinfo> | ||
| − | This part is a combination of a rbs, a mbp, the coding sequence of a mercury metal binding peptide egineered from MerR.It was designed to function as mercury(II) binding peptide in our project.It is expressed in the cytosol and when fused with the periplasm protein Dsba or the membrane protein | + | This part is a combination of a rbs, a mbp, the coding sequence of a mercury metal binding peptide egineered from MerR.It was designed to function as mercury(II) binding peptide in our project.It is expressed in the cytosol and when fused with the periplasm protein Dsba or the membrane protein Lpp-OmpA, it will be expressed and translocated to the periplasm and membrane.In short, this part function as the fundamental metal binding peptide of mercury in our project. |
Revision as of 15:31, 26 October 2010
RBS(B0032)+MBP(mercury metal binding peptide engineered from MerR)
This part is a combination of a rbs, a mbp, the coding sequence of a mercury metal binding peptide egineered from MerR.It was designed to function as mercury(II) binding peptide in our project.It is expressed in the cytosol and when fused with the periplasm protein Dsba or the membrane protein Lpp-OmpA, it will be expressed and translocated to the periplasm and membrane.In short, this part function as the fundamental metal binding peptide of mercury in our project.
Description
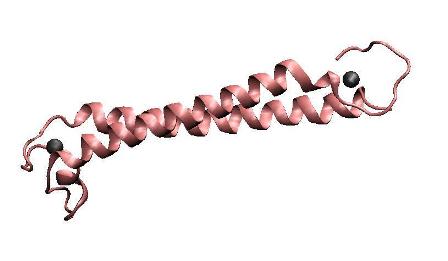
MerR, the mercury-responsible transcription factor(figure on the left), acts as an effective mercury accumulator in aquatic environment.However, as a transcription regulator, over-expression of MerR in bacteria may lead to some unpredictable side effect. Earlier work suggested that the truncated peptide only consisting of the metal binding domain can form a stable dimer with its mercury binding affinity remained and DNA binding domain and metal binding domain can function individually.Based on all these above and carefully structure analysis of MerR via 3D structure modeling, we directly tandemed two copies of metal binding domain of MerR together, to implement a mercury metal binding peptide (MBP) as is shown in the figure on the right.
The figure shows the structure of MerR and MBP. The left figure is MerR, the mercury-responsive transcription factor. The right figure shows the predicted structure of resulted metal binding peptide. Mercury ions are indicated as black balls in metal binding pockets.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 184
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]