Difference between revisions of "Part:BBa K3739010"
(→Characterization of signal peptides) |
|||
| Line 58: | Line 58: | ||
<br/> | <br/> | ||
The data shows that after the protein synthesis was inhibited, the change in the amount of secreted protein over time was well matched with the model. | The data shows that after the protein synthesis was inhibited, the change in the amount of secreted protein over time was well matched with the model. | ||
| + | |||
| + | ===The effect of different promoters on the function of LMT signal peptides=== | ||
| + | ====Construction of gene circuit==== | ||
| + | We constructed circuits driven by promoters of different strengths (J23100, J23103, J23104, J23106, J23110, J23114), each containing RiboJ-B0034-LMT-linker-sfgfp-B0010. The composite part J23104-RiboJ-B0034-LMT-linker-sfgfp-B0010 (BBa_K5136202 ) was used as an example, and the rest results of colony PCR are detailed in <partinfo>BBa_K5136200</partinfo>,<partinfo>BBa_K5136201</partinfo>,<partinfo>BBa_K5136203</partinfo>,<partinfo>BBa_K5136204</partinfo>,<partinfo>BBa_K5136205</partinfo>. | ||
| + | <center><html><img src="https://static.igem.wiki/teams/5136/part/crq-kyh/202.png" width="400px"></html></center> | ||
| + | <br> | ||
| + | <center><b>Figure 8 DNA gel electrophoresis of the colony PCR products of BBa_ K5136202_pSB1C3 </b></center> | ||
| + | |||
| + | ====Characterization of secretion performance==== | ||
| + | By measuring the fluorescence intensity in the supernatant of each circuit, we can quantitatively analyze the secretion of the LMT signal peptide under different promoter strengths and thus evaluate its performance (Figure 9). | ||
| + | <center><html><img src="https://static.igem.wiki/teams/5136/part/lzm/lmt9.png" width="400px"></html></center> | ||
| + | <br> | ||
| + | <center><b>Figure 9 Fluorescence intensity in the supernatant of circuits driven by BBa_K5136200, BBa_K5136201, BBa_K5136202, BBa_K5136203, BBa_K5136204, BBa_K5136205 </b></center> | ||
| + | |||
| + | We find that this follows index kinetics, which is consistent with the T7-mediated secretion. As shown in Figure 10A, secretion efficiency is affected by many factors, but not positively related to promoter strength. The R square (R<sup>2</sup>) indicated that the fitting effect was better, and J23104 (0.9786) was the highest (Figure 3B). | ||
| + | <center><html><img src="https://static.igem.wiki/teams/5136/part/lzm/lmt10.png" width="400px"></html></center> | ||
| + | <br> | ||
| + | <center><b>Figure 10</b> (<b>A</b>) Fitted curve of fluorescence intensity in the supernatant of circuits driven by BBa_K5136200, BBa_K5136201, BBa_K5136202, BBa_K5136203, BBa_K5136204, BBa_K5136205. (<b>B</b>) R square of fit of BBa_K5136200, BBa_K5136201, BBa_K5136202, BBa_K5136203, BBa_K5136204, BBa_K5136205.</center> | ||
| + | |||
| + | To ensure that these results reflect actual secretion rather than differences in cell growth, we measured fluorescence intensity data in 30 h of culture (Figure 11A) and supernatant (Figure 11B) and calculated the secretion efficiency (supernatant/culture) (Figure 11C). | ||
| + | <center><html><img src="https://static.igem.wiki/teams/5136/part/lzm/lmt11.png" width="400px"></html></center> | ||
| + | <br> | ||
| + | <center><b>Figure 11</b> (<b>A</b>) Fluorescence intensity (30 h) of culture driven by BBa_K5136200, BBa_K5136201, BBa_K5136202, BBa_K5136203, BBa_K5136204, BBa_K5136205. (<b>B</b>)Fluorescence intensity (30 h) of culture driven by BBa_K5136200, BBa_K5136201, BBa_K5136202, BBa_K5136203, BBa_K5136204, BBa_K5136205.(<b>C</b>)Secretion Efficiency (30 h) driven by BBa_K5136200, BBa_K5136201, BBa_K5136202, BBa_K5136203, BBa_K5136204, BBa_K5136205.</center> | ||
| + | |||
| + | Interestingly, when we compared the secretion efficiency at 30 hours, we found that high secretion efficiency does not mean high protein content in supernatant and culture. Although the secretion efficiency of the weak promoter and the strong promoter were both higher, the weak promoter could fully realize extracellular secretion due to its low efficiency and less protein production by the exosystem, but the amount of protein in supernatant was less. For strong promoters, although the amount of protein secreted is large, the total fluorescence is low, indicating that the metabolic burden is heavy, which affects the survival rate. If the detection continues, very few bacteria will survive. Also, we use a multi-scale model to deconstruct the dynamic secretion process of signal peptides accurately. The experimental results aligned well with the model's predictions (see Model for more details). By trading off, BBa_K5136202 with promoter J23104 has the most appropriate secretion efficiency. | ||
===Reference=== | ===Reference=== | ||
Latest revision as of 11:28, 2 October 2024
LMT-his-GFP
This part contains a Vibrio natriegens-optimized GFP coding sequence, and the GFP is fused at N-terminal with his-tag in order to let us purify the protein. His-GFP is fused with secretory peptide from LMT.We use BBa_K3739010 to construct the expression system and help prove the function of some signal peptide.
Biology
LMT
Lytic murein transglycosylase (LMT) is an enzyme which is able to degrade murein, a component of cell wall of bacteria. There are two kind of LMTs existing in E.coli: the membrane-binding one and the soluble one. The gene coding LMT homolog is also incorporated in the genome of Vibrio natriegens. The LMT signal peptide (named LMT in our parts) is from the LMT homolog, which can lead the fused protein secreted out of Vibrio natriegens.
GFP
Protein tags are peptides genetically fused to the proteins of interest. There are many different kinds of tags developed for for different purposes. For labeling experiments, normally the proteins of interest are tagged directly by fluorescent tags. Green fluorescent protein (GFP) make it easier to study protein localization, interactions and dynamics when fusing with other peptides and proteins.
Usage
Here, we used BBa_K3739003 to construct the expression system and obtained the composite part BBa_K3739043. We transformed the constructed plasmid on the expression vector pET-28a (+) into V. natriegens to extract the protein. This protein work together with those from BBa_K3739038 and BBa_K3739041 to verify the function of our secretory peptides Aly01 and LMT.
Characterization
Colony PCR
We construct BBa_K3739010 with J23100 promotor, B0030 RBS and B0010, the transformed cells are selected by colony PCR. The experiment result is showed in Fig. 1.

Fig. 1. DNA gel electrophoresis of colony PCR product of J23100-B0030-LMT-his-GFP-pSB1C3
Characterization of signal peptides
Since some proteins in our project are intended to function in extracellular environment, how to get out of the cells is vital for realization of their functions. Signal peptide is a short amino acid sequence on secreted protein, which is a natural helper of protein to get out of cytoplasm. However, signal peptides can lead protein go to not only the extracellular environment but also the periplasm and membrane. There are several signal peptides selected for V.natriegens, but these are all directed into periplasm. 1 Although there is a signal peptide of alginate lyase from V.natriegens able to direct heterogenous protein to extracellular environment in E.coli chassis, It is not clear that there is a signal peptide conducting protein to extracellular environment in V.natriegens. 2
Selected procedure
Here, we use a tip to select the signal peptide. Download the translated CDS information of V.natriegens from NCBI Assembly. Through analysis of SignalP 5.0, we found out several candidate signal peptides which have significant likelihood to work with corresponding secretion system. According to the function of proteins containing proposed signal peptide, we can exclude the signal peptides targeting to the periplasm and cell membrane. Finally, we find out the best candidate signal peptide, LMT signal peptide, from our chassis
Qualification results
To clarify whether the signal peptides we selected are able to direct the fused proteins to extracellular environment, we constructed Aly01 and LMT single peptides with BBa_K2560063 which is from 2018 Marhurg iGEM program and transformed the pET28a(+) plasmid with composite parts BBa_K3739038 and BBa_K3739041 into V.natriegens. Then we use the anti-GFP antibody to detect secreted GFP in the filtered supernatant of V.natriegens liquid culture by western blotting after V.natriegens is cultivated for .16 hours. The concentration of his-GFP in the supernatant of BBa_K3739041 is significantly higher than that in J23100-B0030-GFP. (Fig. 2A, 2B) Furthermore, less expression of BBa_K3739041 than that of GFP conforms that LMT-his-GFP is secreted rather than from lysed cells. (Fig. 2C) However, the concentration of Aly01-his-GFP is less than that of GFP. (Fig. 2A, 2B) It is probably because that there are less expression of Aly01-his-GFP in V.natriegens. (Fig. 2C) Thus, we use another method to prove our hypothesis.
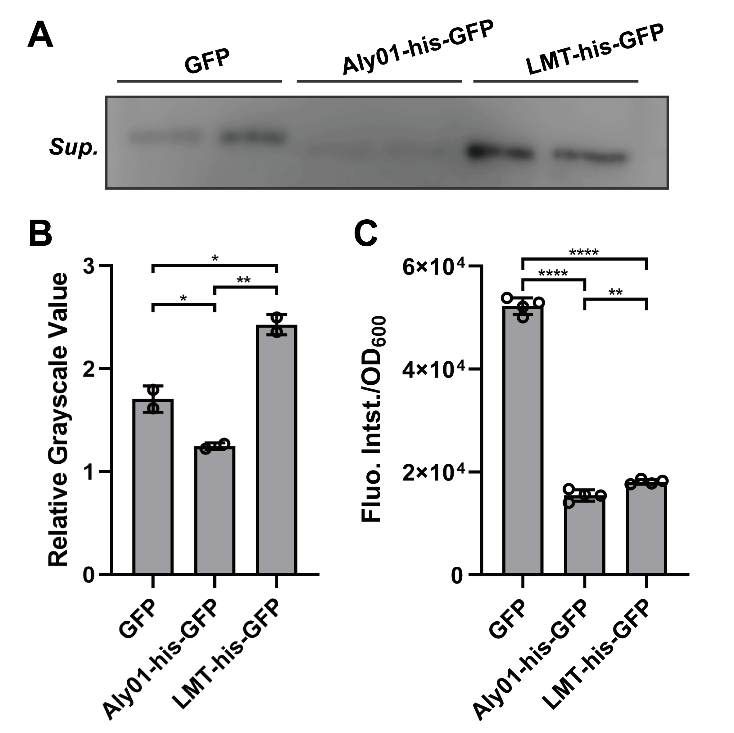
Fig. 2. (A) Western Blotting detection of the V.natriegens supernatants of J23100-B0030-GFP, BBa_K3739038 and BBa_K3739041 after 16h cultivation in LBv2 liquid culture, 37℃. Sup. means supernatant. (B) Grayscale analysis of (A). (C) Fluorescence intensity/OD600 value of V.natriegens strain of J23100-B0030-GFP, BBa_K3739038 and BBa_K3739041 in LBv2 liquid culture after 16h cultivation in 37℃.
Quantitation results
In order to exclude the difference of expression among J23100-B0030-GFP, BBa_K3739038 and BBa_K3739041 and quantify secretion effect of signal peptide, we treated V.natriegens liquid cultures after 12.5 h cultivation in 37℃ with 12.5 μg/mL chloramphenicol. Then we sampled the cultures every 6 minutes and terminated secretion with 20 mM NaN3. As time goes, the his-GFP concentration of culture supernatant were increased in both BBa_K3739038 and BBa_K3739041. This is indicated that both LMT and Aly01 signal peptide are able to translocate fused protein to extracellular environment. (Fig. 3A, 3B) We made a grayscale analysis for the modelling of secretion, and the total GFP is detected for confirmation that the translation is completely repressed. (See our Model https://2021.igem.org/Team:XMU-China/Model pages)
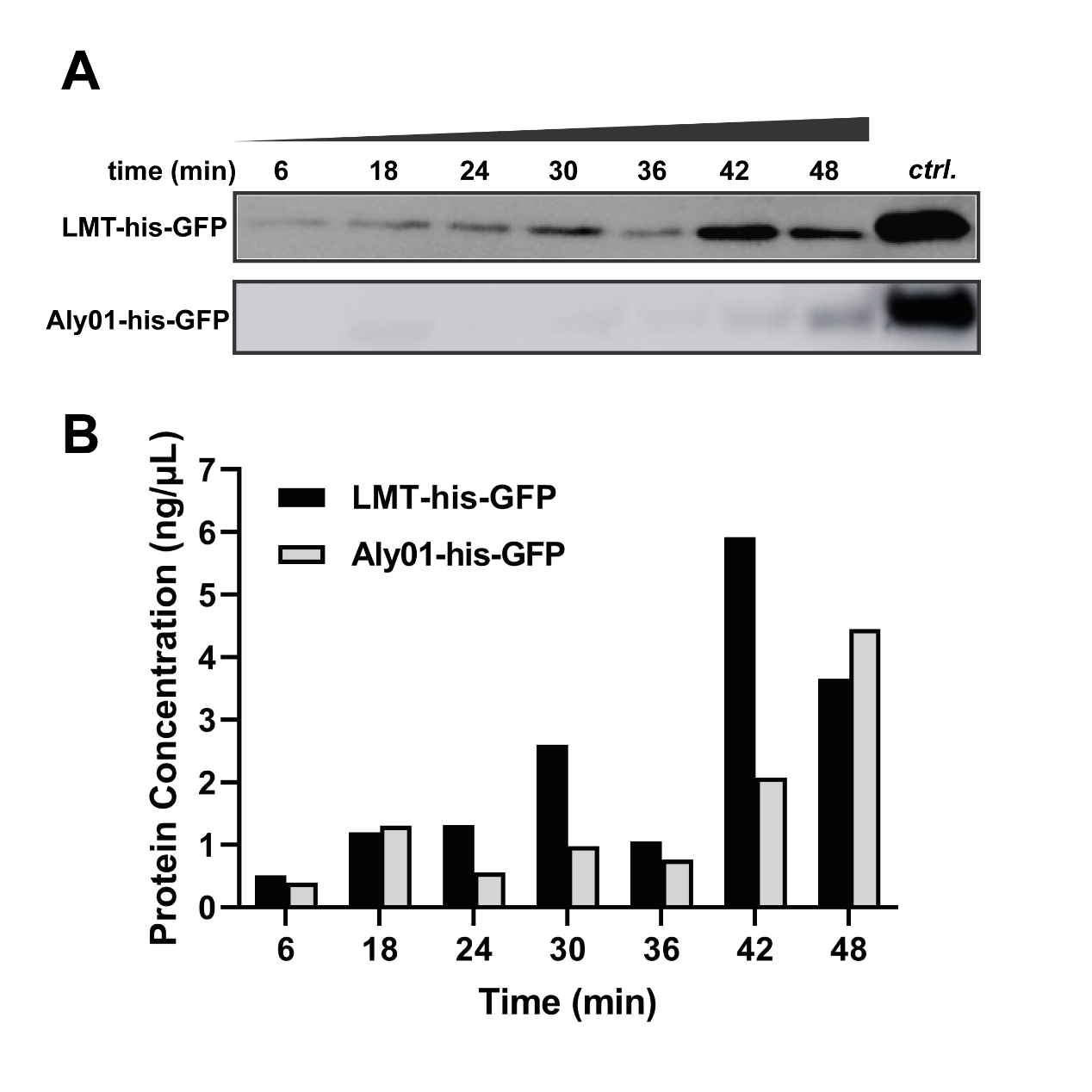
Fig. 3. (A) Western blotting detection of sampled supernatants treated with chloramphenicol in different times. (B) GFP concentration of treated supernatant of BBa_K3739038 and BBa_K3739041 in (A)
Secretion in E.coli
Besides conducting protein secretion in V.natriegens, Aly01 signal peptide is reported that it is able to conduct protein secretion in E.coli as well. 2We supposed that LMT signal peptide may also have conducting function in E.coli. Thus, we used filtered culture of BL21 strain transformed with pET28a(+)-BBa_K3739043 and pET28a(+)-BBa_K3739041 for SDS-PAGE. Then the SDS-PAGE gel is stained by silver staining. Contrasting to BBa_K3739043, his-GFP is detected in the supernatant of pET28a(+)-BBa_K3739041.(Fig. 4) It is indicated that LMT signal peptide can also work for conducting
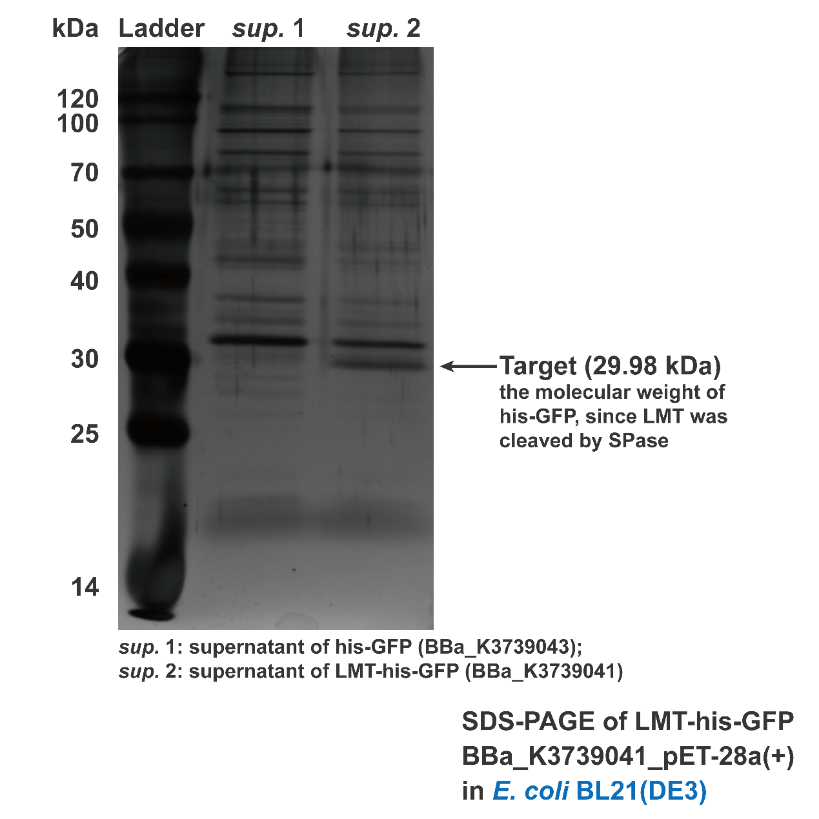
Fig. 4. Silver staining result of supernatant of BL21(DE3) transformed with BBa_K3739043 and BBa_K3739041, respectively.
Then we used an alternative way to verify the secretion function of signal peptides in E.coli. The proteins labeled of tetracysteine motif tag (FlAsH tag, FT in short) could be detected with the biarsenical compound FlAsH-EDT2. 3
To quantify and qualify the secretion effect, FT was added into the C-terminal of BBa_K3739000, BBa_K3739006 and BBa_K3739009. Composite parts BBa_K3739084, BBa_K3739085, BBa_K3739087 were constructed (Fig. 5) on plasmid backbone pSB1C3 or pET-28a(+) and then the recombinant plasmid was transformed into E.coli BL21 (DE3). Positive colonies selected by colony PCR and sequencing were cultivated for secretion analysis.
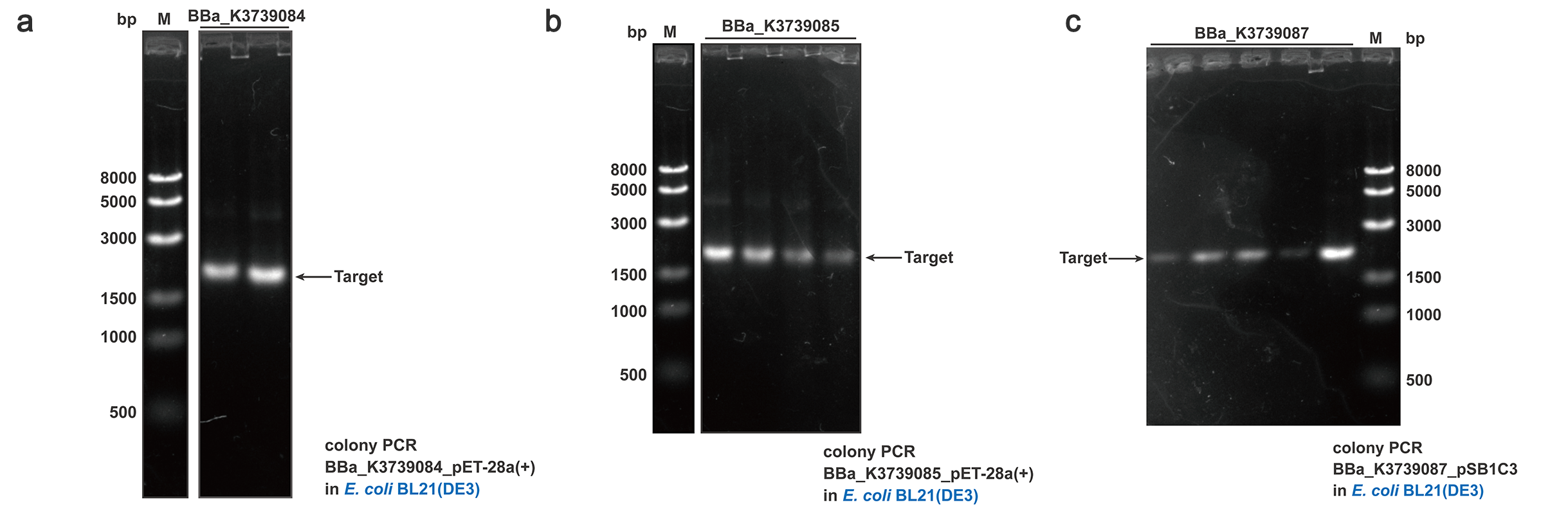
Fig. 5. Colony PCR results of BBa_K3739084, BBa_K3739085, BBa_K3739087
After cultivation for 5 hours at 37℃ and induction for 2 hours with 0.1 mM IPTG, the cell supernatant was separated by centrifugation. The supernatant along with 2 μM FlAsH-EDT2 and 1 mM DTT were added to wells in a 96-well plate. Following incubation in the dark for 1 h at 37 °C, fluorescence was measured by 503 nm excitation and 528 nm emission, and each value was normalized by OD600. The result shows that the fluorescence intensity/OD600 of the group Aly01 and LMT is significantly higher than native control group, which proves that these 2 signal peptides can function well. (Fig. 6)
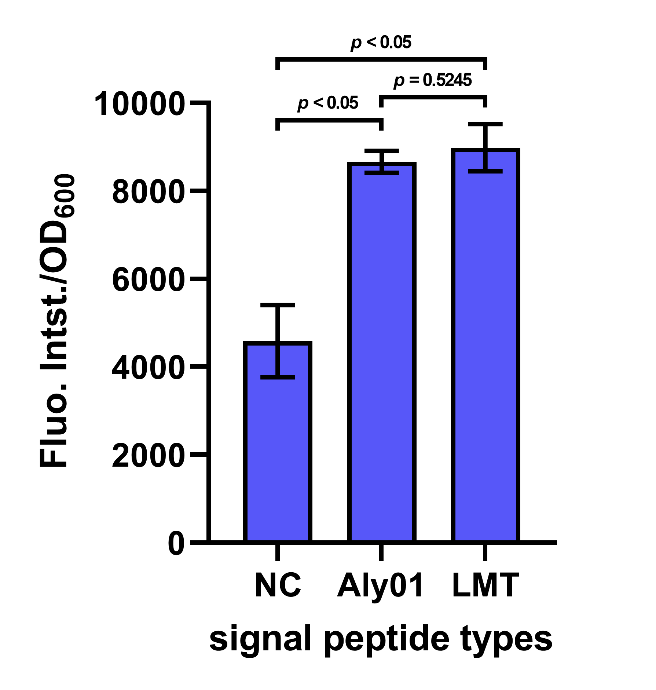
Fig. 6. Fluorescence intensity/OD600 of cell supernatant after incubation in the dark for 1h.
Moreover, to promote our understanding on secretion process mediated by signal peptides, we built a model about relationship between the amount of secreted recombinant protein and time. (See our Model https://2021.igem.org/Team:XMU-China/Model pages) In order to get the value of PT (the total amount of recombinant protein per unit volume of reactor), PM (the amount of the recombinant protein in the medium compartment in unit volume) and γ(extracellular secretion rate constant), a sets of characterization experiments were done.
BBa_K3739087 were constructed on plasmid backbone pET-28a(+) and then the recombinant plasmid was transformed into E.coli BL21 (DE3). After cultivation for 5 hours and induction for 3 hours with 0.1 mM IPTG, 50mg/L chloroamphenicol was added to bog down the synthesis of protein in the engineered bacteria in order to make the experimental operations more convenient. Then, the cell supernatant was collected in 0, 6, 12, 18, 24, 30, 40 min to test the amount of secreted recombinant protein by fluorescence detection of FlAsH-EDT2 and FT. (Fig. 7)
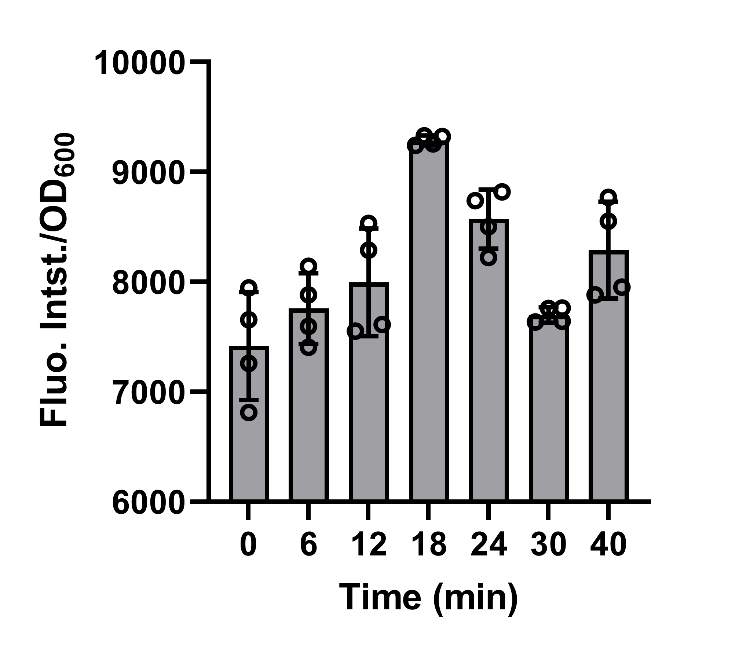
Fig. 7. the fitting of equations and experiments results.
The data shows that after the protein synthesis was inhibited, the change in the amount of secreted protein over time was well matched with the model.
The effect of different promoters on the function of LMT signal peptides
Construction of gene circuit
We constructed circuits driven by promoters of different strengths (J23100, J23103, J23104, J23106, J23110, J23114), each containing RiboJ-B0034-LMT-linker-sfgfp-B0010. The composite part J23104-RiboJ-B0034-LMT-linker-sfgfp-B0010 (BBa_K5136202 ) was used as an example, and the rest results of colony PCR are detailed in BBa_K5136200,BBa_K5136201,BBa_K5136203,BBa_K5136204,BBa_K5136205.

Characterization of secretion performance
By measuring the fluorescence intensity in the supernatant of each circuit, we can quantitatively analyze the secretion of the LMT signal peptide under different promoter strengths and thus evaluate its performance (Figure 9).

We find that this follows index kinetics, which is consistent with the T7-mediated secretion. As shown in Figure 10A, secretion efficiency is affected by many factors, but not positively related to promoter strength. The R square (R2) indicated that the fitting effect was better, and J23104 (0.9786) was the highest (Figure 3B).

To ensure that these results reflect actual secretion rather than differences in cell growth, we measured fluorescence intensity data in 30 h of culture (Figure 11A) and supernatant (Figure 11B) and calculated the secretion efficiency (supernatant/culture) (Figure 11C).

Interestingly, when we compared the secretion efficiency at 30 hours, we found that high secretion efficiency does not mean high protein content in supernatant and culture. Although the secretion efficiency of the weak promoter and the strong promoter were both higher, the weak promoter could fully realize extracellular secretion due to its low efficiency and less protein production by the exosystem, but the amount of protein in supernatant was less. For strong promoters, although the amount of protein secreted is large, the total fluorescence is low, indicating that the metabolic burden is heavy, which affects the survival rate. If the detection continues, very few bacteria will survive. Also, we use a multi-scale model to deconstruct the dynamic secretion process of signal peptides accurately. The experimental results aligned well with the model's predictions (see Model for more details). By trading off, BBa_K5136202 with promoter J23104 has the most appropriate secretion efficiency.
Reference
1. Eichmann, J.; Oberpaul, M.; Weidner, T.; Gerlach, D.; Czermak, P., Selection of High Producers From Combinatorial Libraries for the Production of Recombinant Proteins in Escherichia coli and Vibrio natriegens. Frontiers in Bioengineering and Biotechnology 2019, 7.
2. Meng, Q.; Tian, X.; Jiang, B.; Zhou, L.; Chen, J.; Zhang, T., Characterization and enhanced extracellular overexpression of a new salt‐activated alginate lyase. Journal of the Science of Food and Agriculture 2021.
3. Haitjema, C. H.; Boock, J. T.; Natarajan, A.; Dominguez, M. A.; Gardner, J. G.; Keating, D. H.; Withers, S. T.; Delisa, M. P., Universal Genetic Assay for Engineering Extracellular Protein Expression. ACS Synthetic Biology 2014, 3 (2), 74-82.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 133
