Difference between revisions of "Part:BBa K1614000"
| Line 133: | Line 133: | ||
<h4>The DNA concentration response of the T7 polymerase in a chloroplast cell-free system</h4> | <h4>The DNA concentration response of the T7 polymerase in a chloroplast cell-free system</h4> | ||
| − | [[Image:T--Marburg--DNA titration T7 universal test construct.png|thumb|left| | + | [[Image:T--Marburg--DNA titration T7 universal test construct.png|thumb|left|800px|Figure 4:]] |
Revision as of 11:50, 16 October 2021
T7 promoter for expression of functional RNA
Minimal promoter derived from T7 phage promoter.
Usage and Biology
This T7 promoter derivate is especially useful for the use in in vitro applications. The G which is the startpoint for transcription is included in the promoter sequence.
Characterize the T7 promoter – CCU-Taiwan
In our pHMT-LbCas12a construct, we included the T7 promoter (Part:BBa_K1614000) to induce the LbCas12a expression. To characterize the ability of T7 promoter to induce functional RNA, we examined whether the T7 promoter induced LbCas12a RNA can be translated into proteins by SDS-PAGE and Coomassie blue staining. We first transformed pHMT-LbCas12a into E.coli BL21(DE3), which harbors a Lac Operon regulated T7 polymerase. We then expanded the transformed BL21(DE3) cells by TB medium at 37℃. When O.D.600 approach 0.6 ~ 0.8, we induced T7 polymerase expression by adding 0.2 mM IPTG to TB medium and shift BL21(DE3) to 16℃ for 16 hr.
The IPTG treatment increased the T7 polymerase expression in BL21(DE3) as time goes on, which in turn activate T7 promoter. The activated T7 promoter should promote LbCas12 transcription and translation. To examine this, we collected IPTG-induced BL21(DE3) every two hour, and performed SDS-PAGE assay and Coomassie Blue staining to detect LbCas12a protein expression. In brief, 1 ml of BL21(DE3) is harvested every two hour and centrifuged to cell pellet. The cell pellet was then sonicated, and soluble fraction in supernatant was collected by centrifuging at 13200 rpm 30 min. Total 1 ml supernatant, which equal to protein expression in 1ml BL21(DE3), was used in SDS-PAGE and Coomassie Blue staining. To calculate the protein expression at different time points, we quantity the image intensity of LbCas12a protein and BSA standard by Image J.
- Coomassie blue staining shows the LbCas12a protein expression was absent at 0 hr and increased as time goes on. This result indicated that T7 promoter can be activated by IPTG-induced T7 polymerase (Figure 1).
- We then used BSA standard to quantify the LbCas12a protein expression. The simple linear regression of BSA protein expression standard is shown in Figure 2, while the original image intensity detected by Image J is shown in table 1.
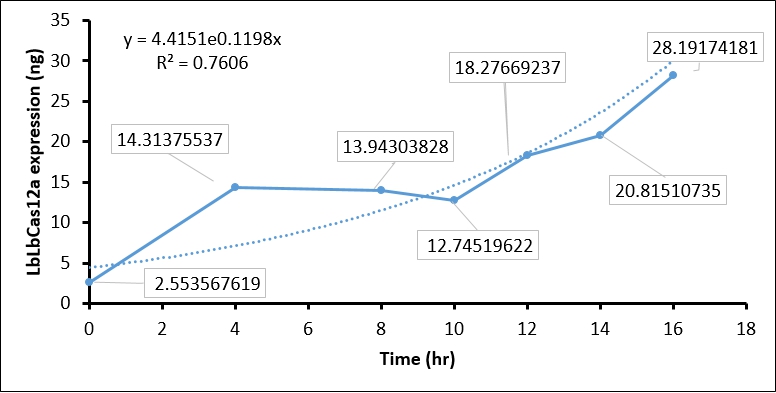
- Finally, we quantify the LbCas12 expression by conversing the intensity to concentration through BSA standard regression (Figure 3).
| BSA (ng) | Background intensity | BSA intensity | BSA - Background intensity |
| 350 | 2407241 | 1258862 | 1148379 |
| 210 | 1589271 | 962468 | 626803 |
| 70 | 1544912 | 904623 | 180999 |
| 35 | 1042670 | 861671 | 129877 |
| Time (hours) | Background intensity | LbCas12a intensity | LbCas12a - Background intensity | LbCas12a (ng) | LbCas12a (µg/mL) |
| 0hr +IPTG | 696645 | 691258 | 5387 | 8.937 | 2.554 |
| 4hr +IPTG | 900536 | 760105 | 140431 | 50.098 | 14.314 |
| 8hr +IPTG | 925618 | 789444 | 136174 | 48.801 | 13.943 |
| 10hr +IPTG | 913959 | 791540 | 122419 | 44.608 | 12.745 |
| 12hr +IPTG | 956220 | 770282 | 185938 | 63.968 | 18.277 |
| 14hr +IPTG | 976568 | 761481 | 215087 | 72.853 | 20.815 |
| 16hr +IPTG | 1064337 | 764543 | 299794 | 98.671 | 28.192 |
| 16hr Ctrl | 924162 | 743361 | 180801 | 62.403 | 17.829 |
Conclusion:
The quantification of LbCas12a protein expression by BSA standard shows that the Cas12 protein is increased at different time points, which confirmed that the T7 promoter is functional in our construct.