Difference between revisions of "Part:BBa K3520012"
m |
|||
| Line 11: | Line 11: | ||
| − | + | =Description= | |
| − | + | ==iGEM KU Istanbul 2020== | |
| + | <br /><br /> | ||
This part was designed during the Partnership of iGEM Athens 2020 and iGEM KU Istanbul 2020. | This part was designed during the Partnership of iGEM Athens 2020 and iGEM KU Istanbul 2020. | ||
The latter team is creating a communication scheme between humans and biological cells by morphing cells into lasers. By this technology, they will be able to detect changes inside and around cells and tissues. These cell lasers can be employed in diagnostics and therapeutic purposes alongside as a high throughput method in basic research. | The latter team is creating a communication scheme between humans and biological cells by morphing cells into lasers. By this technology, they will be able to detect changes inside and around cells and tissues. These cell lasers can be employed in diagnostics and therapeutic purposes alongside as a high throughput method in basic research. | ||
| + | <br><br> | ||
| − | + | ==iGEM Athens 2020== | |
| + | <br /><br /> | ||
iGEM Athens 2020 team during the project MORPHÆ works with Flavobacteriia to produce a non-cellular structurally coloured biomaterial which will require the secretion of a biomolecule that Flavobacteriia do not normally secrete. Our hypothesis is that the formed matrix will have a structure similar to that of the biofilm and thus, it will provide the material with macroscopically the same colouration properties as the biofilm.<br /><br /> | iGEM Athens 2020 team during the project MORPHÆ works with Flavobacteriia to produce a non-cellular structurally coloured biomaterial which will require the secretion of a biomolecule that Flavobacteriia do not normally secrete. Our hypothesis is that the formed matrix will have a structure similar to that of the biofilm and thus, it will provide the material with macroscopically the same colouration properties as the biofilm.<br /><br /> | ||
| Line 24: | Line 27: | ||
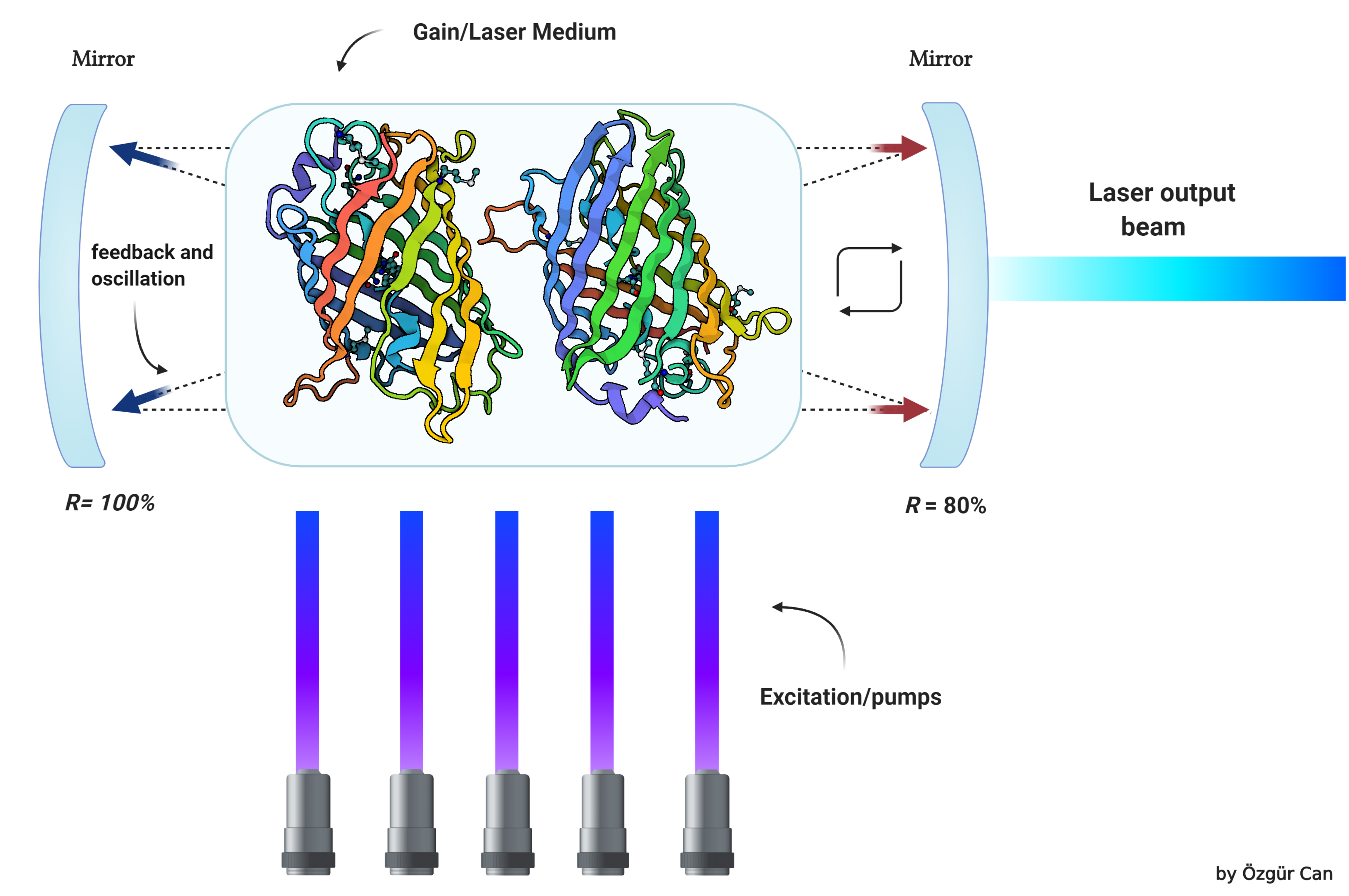
[[File:T--Athens--Instanbul_Collaboration_Biolaser.png|800px|thumb|center|Figure 1: Basics of a biolaser]] | [[File:T--Athens--Instanbul_Collaboration_Biolaser.png|800px|thumb|center|Figure 1: Basics of a biolaser]] | ||
| − | + | ==Further information about Biolaser's Function== | |
| + | <br /><br /> | ||
Biological laser or biolaser is an emerging concept with huge potential in biological and biomedical research. Biolaser is a new type of laser which has biological materials as part of its gain medium or optical feedback. Biolasers are very close to fluorescence microscopy where active molecules (fluorescent) emit light upon excitation by an external laser. However, biolasers are different from fluorescence with respect to optical feedback and gain. The light emitted from a biolaser is coherent, meaning it provides a high signal to noise ratio, consists of a narrow spectrum, and can focus on a small spot, whereas fluorescence radiation is dispersive and very weak compared to biolasers. We are developing a biological laser concept in which no artificial cavities will be used to confine light as opposed to conventional biolasers. Cavities we will be using are composed of natural biological materials which cover the membrane of the cell. So the biolaser concept is fully composed of biological materials. This goal will be achieved by genetically modifying cells to express silicatein and reflectin proteins as well as fluorescent proteins. Expressed silicatein and reflectins will be transported toward the membrane of the cell, and these proteins will be covering the inside and/or outside of the cell. | Biological laser or biolaser is an emerging concept with huge potential in biological and biomedical research. Biolaser is a new type of laser which has biological materials as part of its gain medium or optical feedback. Biolasers are very close to fluorescence microscopy where active molecules (fluorescent) emit light upon excitation by an external laser. However, biolasers are different from fluorescence with respect to optical feedback and gain. The light emitted from a biolaser is coherent, meaning it provides a high signal to noise ratio, consists of a narrow spectrum, and can focus on a small spot, whereas fluorescence radiation is dispersive and very weak compared to biolasers. We are developing a biological laser concept in which no artificial cavities will be used to confine light as opposed to conventional biolasers. Cavities we will be using are composed of natural biological materials which cover the membrane of the cell. So the biolaser concept is fully composed of biological materials. This goal will be achieved by genetically modifying cells to express silicatein and reflectin proteins as well as fluorescent proteins. Expressed silicatein and reflectins will be transported toward the membrane of the cell, and these proteins will be covering the inside and/or outside of the cell. | ||
| − | + | ==Signal Peptide== | |
| + | <br /><br /> | ||
The OPH (Organophosphorus hydrolase) signal peptide is used by Flavobacteriia to localise the OPH, a membrane-associated homodimeric metalloenzyme, capable of hydrolyzing numerous types of organophosphorus compounds (Ops). These compounds contain phosphate-ester bonds such as P-O, P-F, P-CN, and P-S, which are harmful neurotoxins. OPH identified from Flavobacterium species and Brevundimonas diminuta are the most studied enzymes, which are homodimeric phosphotriesterases requiring a zinc ion at their respective catalytic centers[1]. | The OPH (Organophosphorus hydrolase) signal peptide is used by Flavobacteriia to localise the OPH, a membrane-associated homodimeric metalloenzyme, capable of hydrolyzing numerous types of organophosphorus compounds (Ops). These compounds contain phosphate-ester bonds such as P-O, P-F, P-CN, and P-S, which are harmful neurotoxins. OPH identified from Flavobacterium species and Brevundimonas diminuta are the most studied enzymes, which are homodimeric phosphotriesterases requiring a zinc ion at their respective catalytic centers[1]. | ||
| Line 38: | Line 43: | ||
Underlined: twin-arginine motif (consensus sequence: S/T-R-R-X-F-L-K) | Underlined: twin-arginine motif (consensus sequence: S/T-R-R-X-F-L-K) | ||
| − | + | =References= | |
| + | <br /><br /> | ||
[1]Kang, D., Seo, J., Jo, B., Kim, C., Choi, S., & Cha, H. (2016). Versatile signal peptide of Flavobacterium-originated organophosphorus hydrolase for efficient periplasmic translocation of heterologous proteins in Escherichia coli. Biotechnology Progress, 32(4), 848-854. doi: 10.1002/btpr.2274 | [1]Kang, D., Seo, J., Jo, B., Kim, C., Choi, S., & Cha, H. (2016). Versatile signal peptide of Flavobacterium-originated organophosphorus hydrolase for efficient periplasmic translocation of heterologous proteins in Escherichia coli. Biotechnology Progress, 32(4), 848-854. doi: 10.1002/btpr.2274 | ||
Latest revision as of 01:13, 28 October 2020
Signal Peptide for translocation to membrane in Flavobacteriia
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Description
iGEM KU Istanbul 2020
This part was designed during the Partnership of iGEM Athens 2020 and iGEM KU Istanbul 2020.
The latter team is creating a communication scheme between humans and biological cells by morphing cells into lasers. By this technology, they will be able to detect changes inside and around cells and tissues. These cell lasers can be employed in diagnostics and therapeutic purposes alongside as a high throughput method in basic research.
iGEM Athens 2020
iGEM Athens 2020 team during the project MORPHÆ works with Flavobacteriia to produce a non-cellular structurally coloured biomaterial which will require the secretion of a biomolecule that Flavobacteriia do not normally secrete. Our hypothesis is that the formed matrix will have a structure similar to that of the biofilm and thus, it will provide the material with macroscopically the same colouration properties as the biofilm.
So these two teams above, collaborated in a creative way and iGEM Athens designed a cloning experiment in which Flavobacteriia will express reflectin with a signal peptide which will translocate it to the outer membrane and GFP superfolde. As a result, the biolaser designed by iGEM KU Istanbul will be able to track genetically modified Flavobacteriia.
Further information about Biolaser's Function
Biological laser or biolaser is an emerging concept with huge potential in biological and biomedical research. Biolaser is a new type of laser which has biological materials as part of its gain medium or optical feedback. Biolasers are very close to fluorescence microscopy where active molecules (fluorescent) emit light upon excitation by an external laser. However, biolasers are different from fluorescence with respect to optical feedback and gain. The light emitted from a biolaser is coherent, meaning it provides a high signal to noise ratio, consists of a narrow spectrum, and can focus on a small spot, whereas fluorescence radiation is dispersive and very weak compared to biolasers. We are developing a biological laser concept in which no artificial cavities will be used to confine light as opposed to conventional biolasers. Cavities we will be using are composed of natural biological materials which cover the membrane of the cell. So the biolaser concept is fully composed of biological materials. This goal will be achieved by genetically modifying cells to express silicatein and reflectin proteins as well as fluorescent proteins. Expressed silicatein and reflectins will be transported toward the membrane of the cell, and these proteins will be covering the inside and/or outside of the cell.
Signal Peptide
The OPH (Organophosphorus hydrolase) signal peptide is used by Flavobacteriia to localise the OPH, a membrane-associated homodimeric metalloenzyme, capable of hydrolyzing numerous types of organophosphorus compounds (Ops). These compounds contain phosphate-ester bonds such as P-O, P-F, P-CN, and P-S, which are harmful neurotoxins. OPH identified from Flavobacterium species and Brevundimonas diminuta are the most studied enzymes, which are homodimeric phosphotriesterases requiring a zinc ion at their respective catalytic centers[1].
The OPH enzyme is usually located in cell membranes due to its signal peptide. Analysis of OPH proteins has shown that all of them contain a predicted signal peptide harboring a twin-arginine (Tat) motif. Twin-arginine signal peptides serve to target proteins to the twin-arginine protein transport (Tat) pathway, which translocates folded proteins across the bacterial cytoplasmic membrane [1].
MQTRRVVLKSAAAAGTLLGGLAGCASVAGS
Bolded RR: twin-arginine sequence.
Underlined: twin-arginine motif (consensus sequence: S/T-R-R-X-F-L-K)
References
[1]Kang, D., Seo, J., Jo, B., Kim, C., Choi, S., & Cha, H. (2016). Versatile signal peptide of Flavobacterium-originated organophosphorus hydrolase for efficient periplasmic translocation of heterologous proteins in Escherichia coli. Biotechnology Progress, 32(4), 848-854. doi: 10.1002/btpr.2274