Difference between revisions of "Part:BBa K112022"
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K112022 short</partinfo> | <partinfo>BBa_K112022 short</partinfo> | ||
This variation uses PconC5 to drive the production of antiholin. | This variation uses PconC5 to drive the production of antiholin. | ||
| + | |||
| + | |||
| + | ==Characterization== | ||
| + | '''For more info, visit [http://2008.igem.org/Team:UC_Berkeley/LysisDevice UC Berkeley iGEM08 Wiki!!]''' | ||
| + | |||
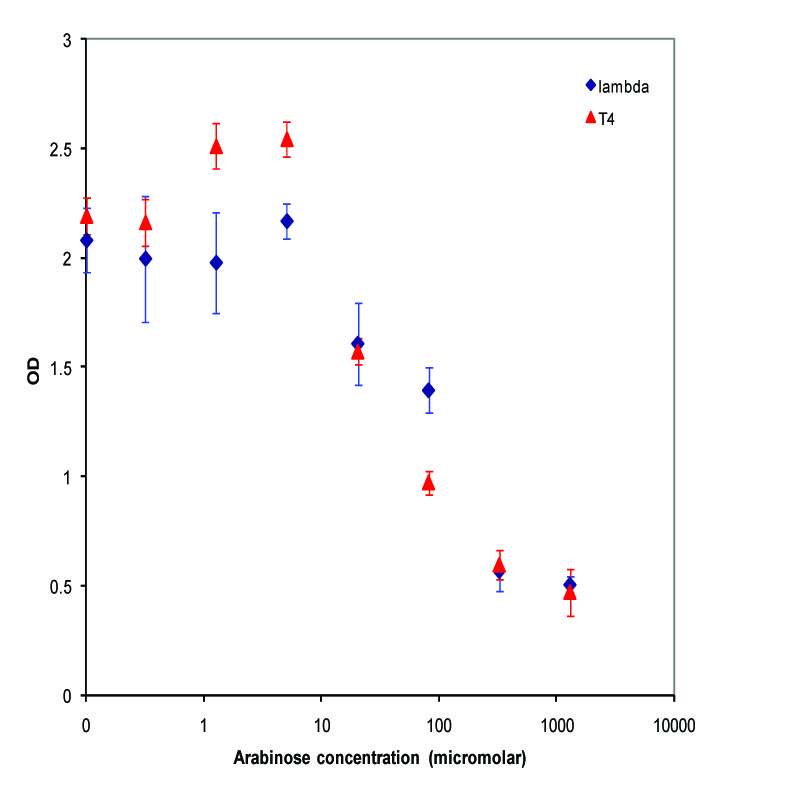
| + | K112022 (Lambda Lysis Device) was assembled with a Pbad promoter and inserted in the vector K112950 and K112809 was inserted in pSB1A2. They were introduced into the MC1061 strain by transformation, and then picked 5 colonies for each device. We grew these cultures to saturation at 37 degrees Celsius in LB media, and then split into eight 1 mL aliquots. A range of concentrations of arabinose was added to these aliquots, with a starting concentration of 1.3E-3 M and the next 6 samples recieving a four-fold dilution of the previous sample, and an equal volume of water added to the last aliquot. The cultures were then incubated at 37 degrees again for 3.5 hours, and the absorbance at 600nm was measured with a Tecan Xfluor4 Safire2 in a Corning Inc. Costar 3603 plate. The data plotted on a log scale is shown below. | ||
| + | |||
| + | |||
| + | [[Image:K112019_data1.jpg|thumbnail|450px|center|Data from adding arabinose at midlog. Concentrations used at each data point from left to right are 0, 2.44E-07, 9.77E-07, 3.91E-06, 1.56E-05, 6.25E-05, 2.50E-04, and 1.00E-03 M]] | ||
| + | |||
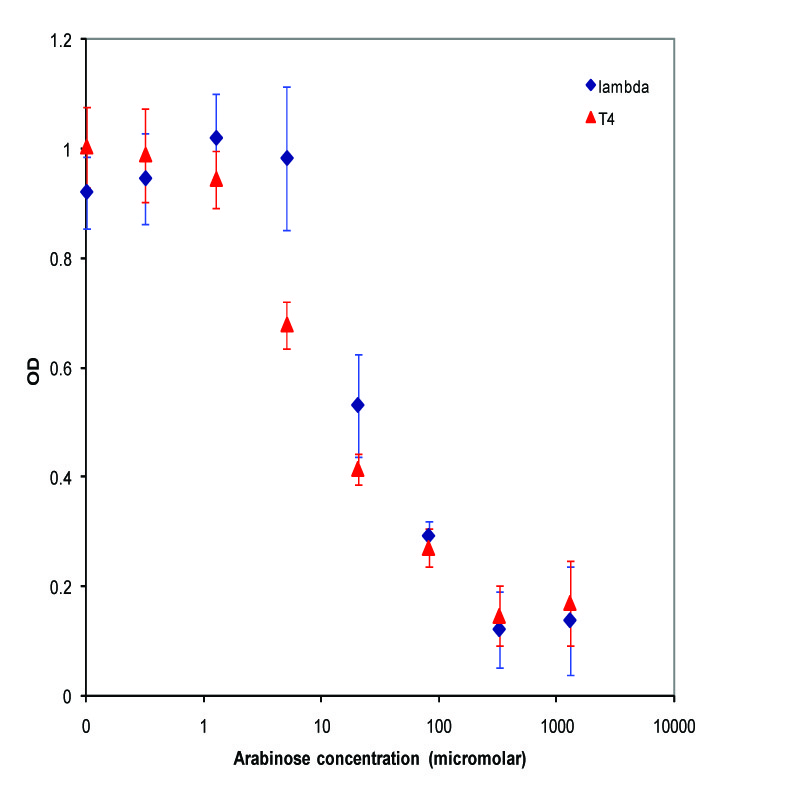
| + | A parallel experiment was performed by taking the same saturated culture before induction with arabinose, diluting 100-fold, growing to mid-log(starting OD .22), before inducing with arabinose as above. The data plotted on a log scale is shown below. | ||
| + | |||
| + | [[Image:K112019_data2.jpg|thumbnail|450px|center|Data from adding arabinose at midlog. Concentrations used at each data point from left to right are 0, 2.44E-07, 9.77E-07, 3.91E-06, 1.56E-05, 6.25E-05, 2.50E-04, and 1.00E-03 M]] | ||
| + | |||
| + | |||
| + | |||
---- | ---- | ||
This part is in BBb Format. It is flanked by BamHI and BglII sites instead of XbaI and SpeI. More information about the BBb Format is available at: | This part is in BBb Format. It is flanked by BamHI and BglII sites instead of XbaI and SpeI. More information about the BBb Format is available at: | ||
Revision as of 09:10, 6 November 2008
Lambda phage lysis device - no promoter
This variation uses PconC5 to drive the production of antiholin.
Characterization
For more info, visit [http://2008.igem.org/Team:UC_Berkeley/LysisDevice UC Berkeley iGEM08 Wiki!!]
K112022 (Lambda Lysis Device) was assembled with a Pbad promoter and inserted in the vector K112950 and K112809 was inserted in pSB1A2. They were introduced into the MC1061 strain by transformation, and then picked 5 colonies for each device. We grew these cultures to saturation at 37 degrees Celsius in LB media, and then split into eight 1 mL aliquots. A range of concentrations of arabinose was added to these aliquots, with a starting concentration of 1.3E-3 M and the next 6 samples recieving a four-fold dilution of the previous sample, and an equal volume of water added to the last aliquot. The cultures were then incubated at 37 degrees again for 3.5 hours, and the absorbance at 600nm was measured with a Tecan Xfluor4 Safire2 in a Corning Inc. Costar 3603 plate. The data plotted on a log scale is shown below.
A parallel experiment was performed by taking the same saturated culture before induction with arabinose, diluting 100-fold, growing to mid-log(starting OD .22), before inducing with arabinose as above. The data plotted on a log scale is shown below.
This part is in BBb Format. It is flanked by BamHI and BglII sites instead of XbaI and SpeI. More information about the BBb Format is available at:
[http://openwetware.org/wiki/Template:AndersonLab:BBb_Standard BBb Standard Description Page]
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 999
Illegal NheI site found at 1022 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1385
- 1000COMPATIBLE WITH RFC[1000]