Difference between revisions of "Part:BBa K823038"
(→Characterization: TU_Dresden 2019) |
(→Strep-tag column purification) |
||
| Line 76: | Line 76: | ||
==== Strep-tag column purification ==== | ==== Strep-tag column purification ==== | ||
| − | The Team TU Dresden 2019 used this Biobrick | + | The Team TU Dresden 2019 used this Biobrick for purification purposes using the “Expression and purification of proteins using Strep-Tactin” protocol of IBA Lifescience. We tried to purify many of our different BioBricks via this method, in order to characterize the proper activity of the Strep-tag for purification purposes. However, we were not able to obtain successfully purified constructs, meaning this that this Strep-tag should rather be used for Western Blotting instead of for purification. The different Biobricks that we tried to purify with this Strep-tag have shown to be working properly (see their Registries: [https://parts.igem.org/Part:BBa_K3037003 BBa_K3037003] , [https://parts.igem.org/Part:BBa_K3037003 BBa_K3037009] ) |
| − | + | The results of the different purification tests are shown in the following gels: | |
| − | + | ||
| − | + | ||
| − | + | ==== Purification of our Full Construct ([https://parts.igem.org/Part:BBa_K3037003 BBa_K3037003]) ==== | |
| − | + | [[File:Strep-tag_purification_BBa_3037003.png|center|400px|thumb|none|Different Full Construct samples before, while and after Strep purification]] | |
| − | |||
| − | |||
| − | |||
| − | + | The purification of this construct was repeated again by using the same protocol, but this time a successful MBP-tag purification was performed first (see the registry page of our Full Construct for more details [https://parts.igem.org/Part:BBa_K3037003 BBa_K3037003]). As shown in the gel, the Strep-tag column purification did not work. | |
[[File:Strep_purification_2.png|center|400px|thumb|none|Different Full Construct samples before, while and after Strep purification]] | [[File:Strep_purification_2.png|center|400px|thumb|none|Different Full Construct samples before, while and after Strep purification]] | ||
| + | |||
| + | |||
| + | ==== Purification of HRP-strep ([https://parts.igem.org/Part:BBa_K3037009]) ==== | ||
| + | |||
| + | |||
| + | For the purification of this construct the same protocol mentioned above was used, however, the same buffers were made by us without adding EDTA to ensure the proper working of the HRP. | ||
| + | |||
| + | |||
| + | [[File:Strep-tag_purification_BBa_3037009.png|center|400px|thumb|none|Different Full Construct samples before, while and after Strep purification]] | ||
==== Conclusions ==== | ==== Conclusions ==== | ||
Revision as of 17:29, 21 October 2019
Strep-tag (Freiburg standard+RBS)
Streptavidin - tag with RBS in Freiburg standard.
Find out more about the design of our prefix with ribosome binding site.
prefix:GAATTCCGCGGCCGCTTCTAGATAAGGAGGAACTACTATGGCCGGC
suffix:ACCGGTTAATACTAGTAGCGGCCGCTGCAGT
The Strep-tag is a mimicry peptide of biotin which binds to Streptavidin ([http://www.sciencedirect.com/science/article/pii/S1050386299000339 Skerra, A. and Schmidt, T.G.M. (1999)]). Its sequence is WSHPQFEK. It can be used for protein purification, immobilisation with Streptavidin or Strep-tactin ([http://www.ncbi.nlm.nih.gov/pubmed/9415448 Voss, S. and Skerra, A. (1997)]) or detection with Strep-tactin or antibodies.
This is a part created by the LMU-Munich 2012 team. We added five tags to the registry, all in the Freiburg standard for N-and C-terminal fusions:
- Strep - tag
Visit our project page for more usefull parts of our [http://2012.igem.org/Team:LMU-Munich/Bacillus_BioBricks BacillusBioBrickBox]. This part was also evaluated in the publication [http://www.jbioleng.org/content/7/1/29 The Bacillus BioBrick Box: generation and evaluation of essential genetic building blocks for standardized work with Bacillus subtilis] by Radeck et al..
Evaluation
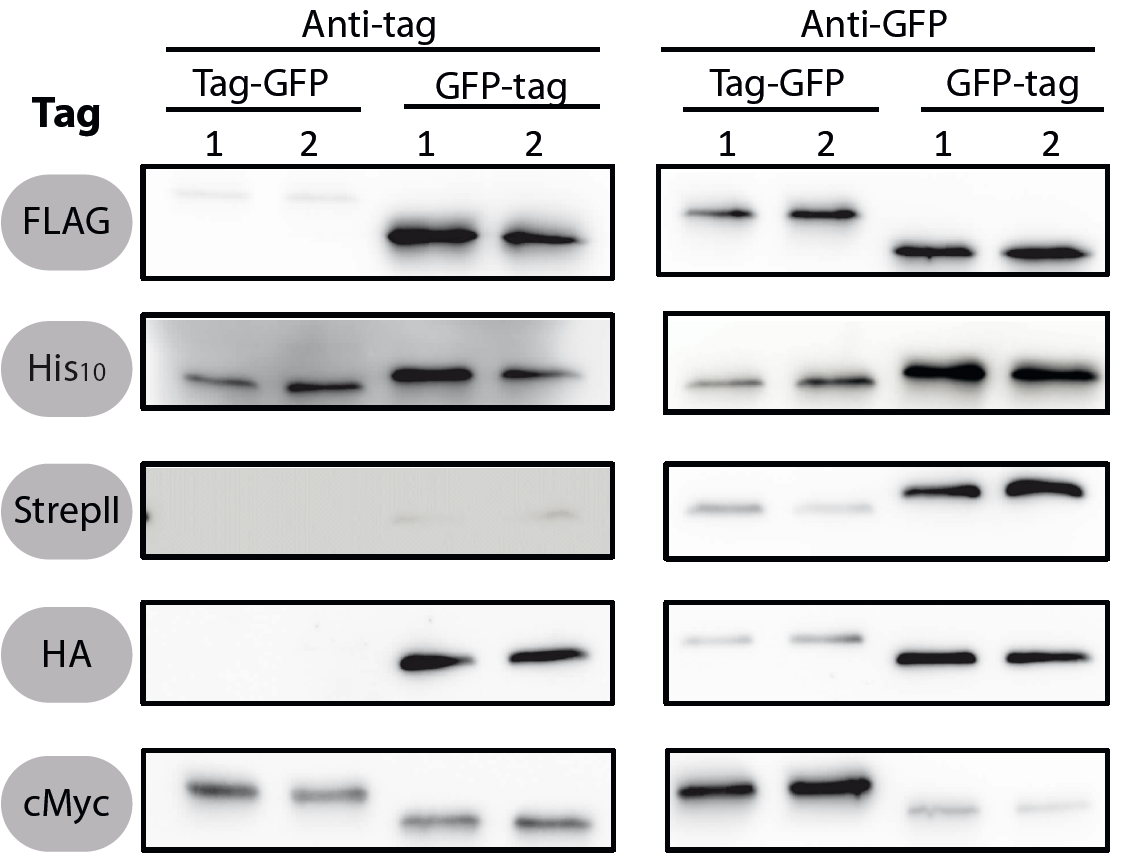
All 5 epitope tags were fused C- and N-terminally to GFP using the NgoMIV and AgeI restriction sites. These constructs were expressed in Bacillus subtils using pSBBs0K-Pspac. This vector did not need to be induced by IPTG due to a premature stop codon in the lacI gene.
|
Methods
To verify the functionality of the epitope tags, Western blot analyses of the strains TMB1920-TMB1929 were performed. LB medium (15 ml) was inoculated 1:100 from overnight culture and grown at 37°C and 200 rpm to OD600 ~ 0.5. Of this, 10 ml were harvested by centrifugation (8000 × g, 5 min) and the pellets stored at -20°C. Pellets were resuspended in 1 ml disruption buffer (50 mM Tris–HCl pH 7.5, 100 mM NaCl) and lysed by sonication. Samples (12 μl of lysate) were loaded per lane on two 12.5% SDS-polyacrylamide gels and SDS-PAGE was performed according standard procedure [60]. One gel was stained with colloidal coomassie, the other one was used for protein transfer to a PVDF membrane (Merck Millipore, Billerica, MA, USA) by submerged blotting procedure (Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA, USA)). After protein transfer, the membranes were treated with the following antibodies and conditions. Detailed protocols can be found [http://www.jbioleng.org/content/7/1/29/suppl/S3 here].
GFP
Probing with primary antibodies takes place with rabbit anti-GFP antibodies (1:3000, Epitomics, No. 1533). Horseradish-peroxidase (HRP)-conjugated anti-rabbit antibodies (1:2000, Promega, W401B) were used as secondary antibody. Hybridization of both antibodies was carried out in Blotto-buffer (2.5% (w/v) skim milk powder, 1 × TBS (50 mM Tris–HCl pH 7.6, 0.15 M NaCl)).
StrepII
Strep-Tactin-HRP conjugate (IBA, Strep-Tactin-HRP conjugate, No. 2-1502-001) 1:100 in 1 × PBS (4 mM KH2PO4; 16 mM Na2HPO4; 115 mM NaCl) with 0.1% (w/v) Tween20 was used.
Chemiluminescence signals were detected after addition of the HRP-substrate Ace Glow (Peqlab, Erlangen, Germany) using a FusionTM imaging system (Peqlab).
Characterization: TU_Dresden 2019
Strep-tag column purification
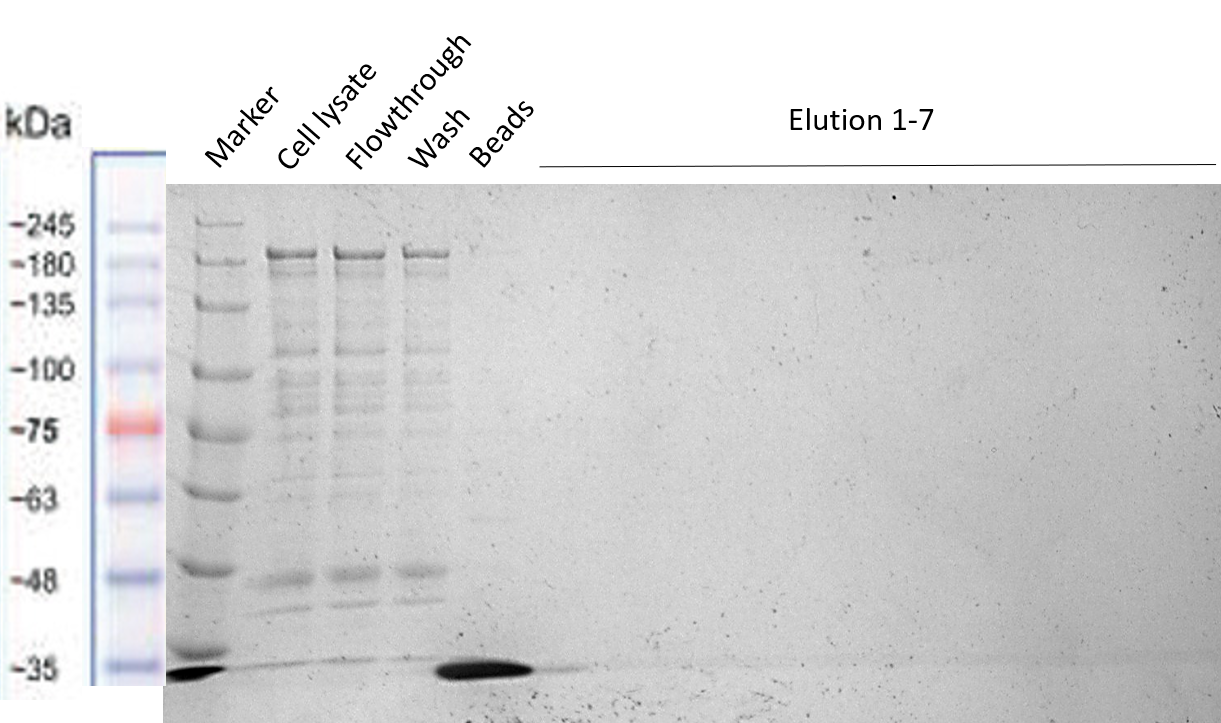
The Team TU Dresden 2019 used this Biobrick for purification purposes using the “Expression and purification of proteins using Strep-Tactin” protocol of IBA Lifescience. We tried to purify many of our different BioBricks via this method, in order to characterize the proper activity of the Strep-tag for purification purposes. However, we were not able to obtain successfully purified constructs, meaning this that this Strep-tag should rather be used for Western Blotting instead of for purification. The different Biobricks that we tried to purify with this Strep-tag have shown to be working properly (see their Registries: BBa_K3037003 , BBa_K3037009 )
The results of the different purification tests are shown in the following gels:
Purification of our Full Construct (BBa_K3037003)
The purification of this construct was repeated again by using the same protocol, but this time a successful MBP-tag purification was performed first (see the registry page of our Full Construct for more details BBa_K3037003). As shown in the gel, the Strep-tag column purification did not work.
Purification of HRP-strep ([1])
For the purification of this construct the same protocol mentioned above was used, however, the same buffers were made by us without adding EDTA to ensure the proper working of the HRP.
Conclusions
From the results of the purifications we conclude that the Strep-tag doesn't works for column purification, and should be used only for Western Blots as it was meant to be.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]