Difference between revisions of "Part:BBa K3171173"
| Line 4: | Line 4: | ||
Improvisation of the pTET promoter to enhance its strength in E. coli. Use of synthetic promoter library on pTet [[Part:BBa_R0040]] in order to enhance function and inducibility. | Improvisation of the pTET promoter to enhance its strength in E. coli. Use of synthetic promoter library on pTet [[Part:BBa_R0040]] in order to enhance function and inducibility. | ||
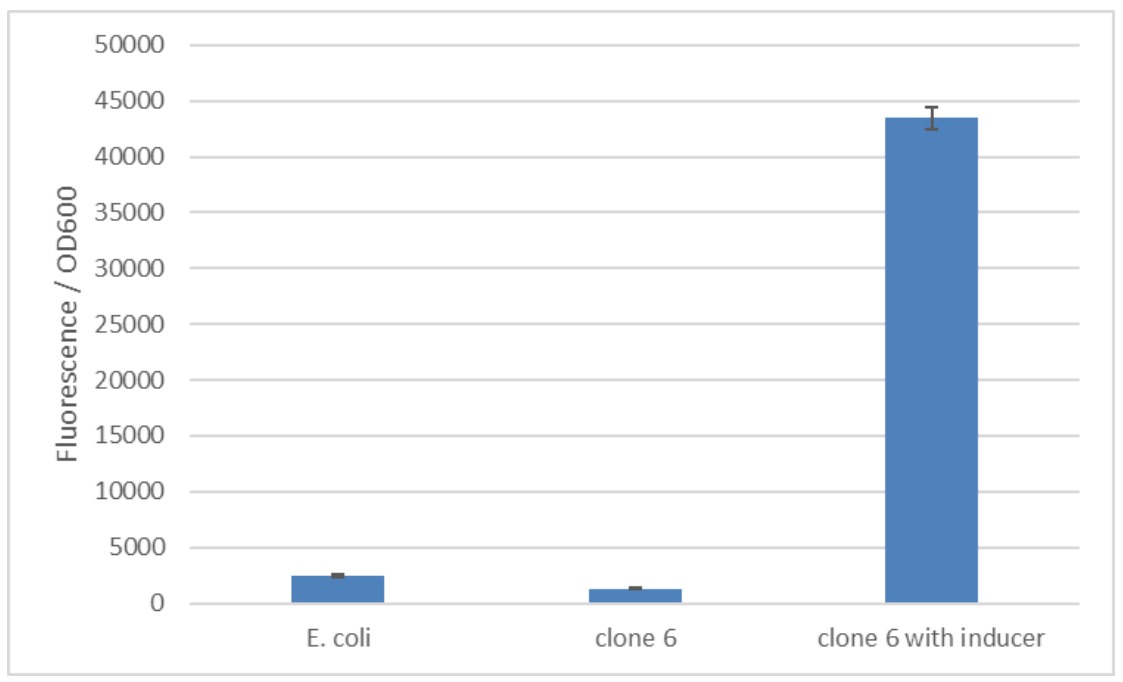
| − | The SPL was constructed using a PCR with randomized primers to yield arbitrary sequences in the promoter region. A total of 245 clones were screened to find a target with enhanced functionality. The cells were induced with 125, 250 and 500ng/mL anhydrous tetracycline. The expression of mCherry was determined with the help of the fluorescence level emitted by it. These fluorescence levels were measured at different time points of 0, 4, 8 and 12 hours after induction. The screening of the final 6 interesting variations revealed clone 6 as the sequence with increased inducibility (figure | + | The SPL was constructed using a PCR with randomized primers to yield arbitrary sequences in the promoter region. A total of 245 clones were screened to find a target with enhanced functionality. The cells were induced with 125, 250 and 500ng/mL anhydrous tetracycline. The expression of mCherry was determined with the help of the fluorescence level emitted by it. These fluorescence levels were measured at different time points of 0, 4, 8 and 12 hours after induction. The screening of the final 6 interesting variations revealed clone 6 as the sequence with increased inducibility (figure 1). The screening was done based on quantifications of the fluorescence intensity measured. The best version of the promoter was determined based on fold change in the fluorescence intensity and low basal level. We calculated a fold change of 34 comparing fluorescence in the induced and uninduced state! Additionally, this promoter also exhibited a low basal level of fluorescence that is comparable to background fluorescence of E. coli without any reporter (figure 2). |
[[File:BBa_K3171173.png|500px|]] | [[File:BBa_K3171173.png|500px|]] | ||
Revision as of 10:42, 21 October 2019
Improved pTet promoter in E. coli
Improvisation of the pTET promoter to enhance its strength in E. coli. Use of synthetic promoter library on pTet Part:BBa_R0040 in order to enhance function and inducibility. The SPL was constructed using a PCR with randomized primers to yield arbitrary sequences in the promoter region. A total of 245 clones were screened to find a target with enhanced functionality. The cells were induced with 125, 250 and 500ng/mL anhydrous tetracycline. The expression of mCherry was determined with the help of the fluorescence level emitted by it. These fluorescence levels were measured at different time points of 0, 4, 8 and 12 hours after induction. The screening of the final 6 interesting variations revealed clone 6 as the sequence with increased inducibility (figure 1). The screening was done based on quantifications of the fluorescence intensity measured. The best version of the promoter was determined based on fold change in the fluorescence intensity and low basal level. We calculated a fold change of 34 comparing fluorescence in the induced and uninduced state! Additionally, this promoter also exhibited a low basal level of fluorescence that is comparable to background fluorescence of E. coli without any reporter (figure 2).
Figure 1: Clone 6 exhibits the highest fluorescence fold change upon induction. Single clones from the synthetic promoter library were induced with 250 ng/ml anhydrotetracycline. The fluorescence fold change is calculated by dividing the fluorescence in the induced state by fluorescence in the absence of inducer. The used fluorescence reporter is mCherry.
Figure 2: The basal level of reporter expression is comparable to E. coli without the construct. Clone 6 from the synthetic promoter library was induced with 250 ng/ml anhydrotetracycline. The used fluorescence reporter is mCherry.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]