Difference between revisions of "Part:BBa K3078005"
| Line 20: | Line 20: | ||
<h1>'''2. Characterization'''</h1> | <h1>'''2. Characterization'''</h1> | ||
| − | <h4>'''2.1 Validation of | + | <h4>'''2.1 Validation of LL-37 construction'''</h4> |
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
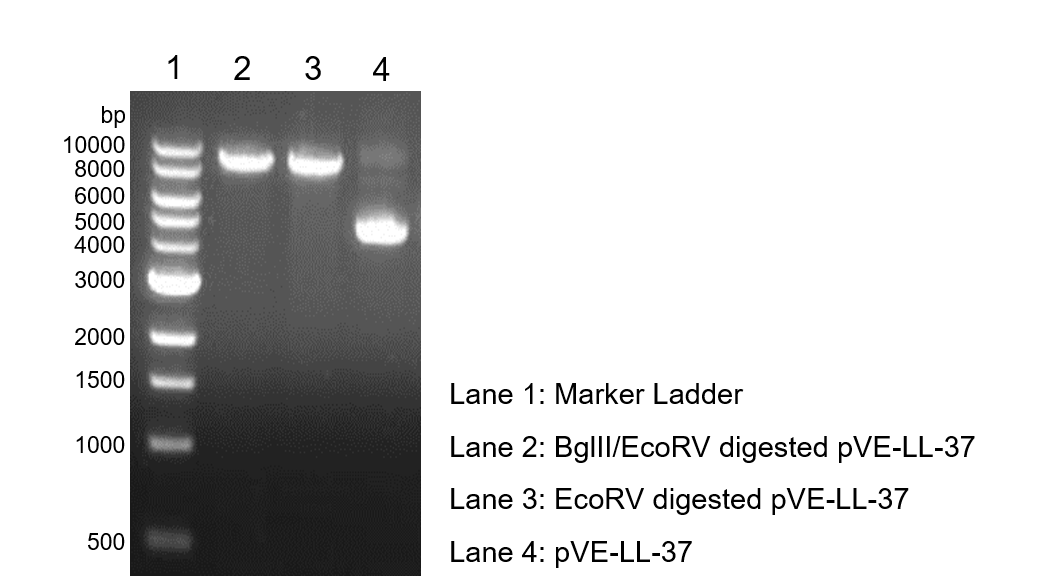
| − | To verify the construction of pVE-LL-37 which we generated, the digestion by BglII/EcoRV was performed by a standard protocol | + | To verify the construction of pVE-LL-37 which we generated, the digestion by BglII/EcoRV was performed by a standard protocol followed by agarose gel electrophoresis (Figure 1). |
</p> | </p> | ||
</h5> | </h5> | ||
| − | [[File: | + | [[File:ll371.png|800px|center|ll37-1]] |
<center> | <center> | ||
| − | Figure 1. Digestion and | + | Figure 1. Digestion and electrophoresis of pVE-LL-37. |
</center> | </center> | ||
| Line 37: | Line 37: | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
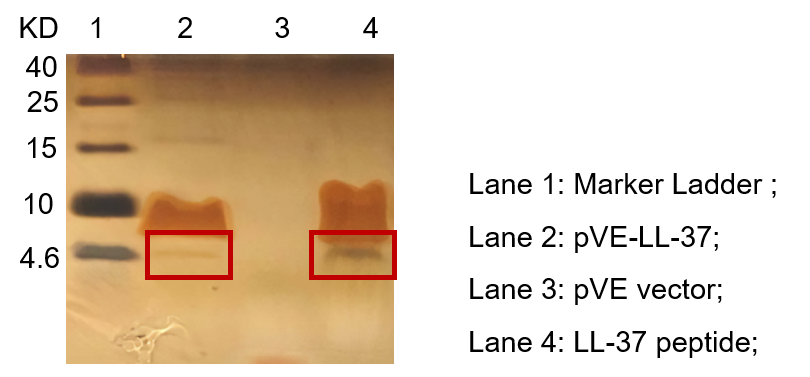
| − | To detect the expression of pVE-LL-37 in E. coli, the constructs were transformed into BL21. Compared to the mock, there was a small size band | + | To detect the expression of pVE-LL-37 in E. coli, the constructs were transformed into BL21. Compared to the mock, there was a small size band shown in pVE-LL-37 (Figure 2). |
</p> | </p> | ||
</h5> | </h5> | ||
| − | [[File: | + | [[File:ll37-2.png|center|ll37-2]] |
<center style="text-align:left;"> | <center style="text-align:left;"> | ||
| − | Figure 2. Expression of pVE-LL-37. The bacteria were collected and | + | Figure 2. Expression of pVE-LL-37. The bacteria were collected and ultrasonicated. The lysate was centrifuged and supernate was electrophoresed on the Tricine-SDS-PAGE gel, followed by Fast silver staining. The LL-37 peptide was synthesized as positive control. |
</center> | </center> | ||
| − | <h4>'''2.3 Candidacidal | + | <h4>'''2.3 Candidacidal effect of LL-37 '''</h4> |
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
| Line 52: | Line 52: | ||
</p> | </p> | ||
</h5> | </h5> | ||
| − | [[File: | + | [[File:ll37-3.png|center|ll37-3]] |
<center style="text-align:left;"> | <center style="text-align:left;"> | ||
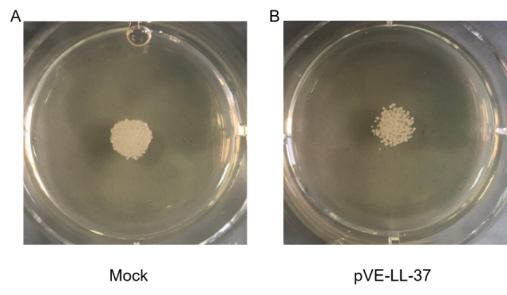
| − | Figure 3. Candidacidal activity of LL-37. C. albicans were collected and suspensions were resuspended to 105 cells/ | + | Figure 3. Candidacidal activity of LL-37. C. albicans were collected and suspensions were resuspended to 105 cells/mL. 10 µL C. albicans suspension and 20 µL sterile BL21-transformed bacteria were gently mixed. After a 10 min incubation, the mixture was spotted onto the SDA agar plates. Cell viability was detected after incubation at 30℃ for 18 hrs. |
</center> | </center> | ||
<h5> | <h5> | ||
<P style="text-indent:2em;"> | <P style="text-indent:2em;"> | ||
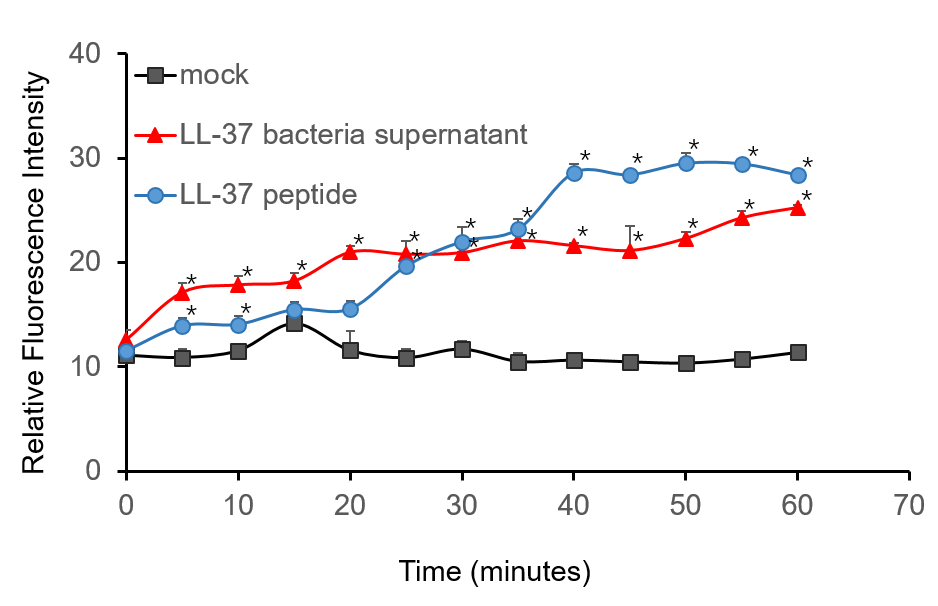
| − | To further validate the | + | To further validate the candidacidal effect of LL-37, C. albicans suspensions were incubated with it. After incubation, LL-37 caused an immediate increase in PI fluorescence indicates that LL-37 can kill C. albicans (Figure 4). |
</p> | </p> | ||
</h5> | </h5> | ||
| − | [[File: | + | [[File:ll37-4.png|center|ll37-4]] |
<center style="text-align:left;"> | <center style="text-align:left;"> | ||
| − | Figure 4. Candidacidal activity of LL-37. 1.5 µM PI (propidiumiodide) was added into C. albicans suspensions (106 cells/ | + | Figure 4. Candidacidal activity of LL-37. 1.5 µM PI (propidiumiodide) was added into C. albicans suspensions (106 cells/mL, 1 mL) and serially BL21-transformed bacteria supernatant (2 mL) mixture. The whole system was incubated at 30 ℃. During the 1 h incubation, the fluorescence was measured every 5 min at the excitation wavelength λexc 544 nm and emission wavelength λem 620 nm. The experiment was performed three times in triplicate. *, P < 0.05 from control using Student’s t test. |
</center> | </center> | ||
Revision as of 15:53, 21 October 2019
LL-37
LL-37 protein coding region. We added a stop codon to BBa_K1162006.
1. Usage and Biology
LL-37 is an antimicrobial peptide (AMP) from the cathelicidin family of antimicrobial peptides, isolated from the Human (Homo sapiens). It is a 37-residue helical peptide found throughout the human body, exhibiting a broad spectrum of antimicrobial activity as well as a variety of immunomodulating effects (Dürr, Ulrich H.N., et. al 2006). It has the distinction of being the first and only cathelicidin member discovered from humans, and is extremely well studied and characterized for this reason. The peptide is produced via epithelial cells as well as leukocytes in places such as the skin, gastrointestinal tract, and the respiratory tract. This AMP is is cleaved from a larger protein, hCAP-18, in a series of modifications to yield its active form (Sorensen, O.E, et. al 2001).
2. Characterization
2.1 Validation of LL-37 construction
To verify the construction of pVE-LL-37 which we generated, the digestion by BglII/EcoRV was performed by a standard protocol followed by agarose gel electrophoresis (Figure 1).
Figure 1. Digestion and electrophoresis of pVE-LL-37.
2.2 Expression of pVE-LL-37
To detect the expression of pVE-LL-37 in E. coli, the constructs were transformed into BL21. Compared to the mock, there was a small size band shown in pVE-LL-37 (Figure 2).
Figure 2. Expression of pVE-LL-37. The bacteria were collected and ultrasonicated. The lysate was centrifuged and supernate was electrophoresed on the Tricine-SDS-PAGE gel, followed by Fast silver staining. The LL-37 peptide was synthesized as positive control.
2.3 Candidacidal effect of LL-37
To assess the candidacidal activity of LL-37, the spot assay was performed. As shown in Figure 3, C. albicans viability after LL-37 treatment was visually compared with that of control cells (no LL-37 treatment). We found that cell viability was more sensitive to LL-37.
Figure 3. Candidacidal activity of LL-37. C. albicans were collected and suspensions were resuspended to 105 cells/mL. 10 µL C. albicans suspension and 20 µL sterile BL21-transformed bacteria were gently mixed. After a 10 min incubation, the mixture was spotted onto the SDA agar plates. Cell viability was detected after incubation at 30℃ for 18 hrs.
To further validate the candidacidal effect of LL-37, C. albicans suspensions were incubated with it. After incubation, LL-37 caused an immediate increase in PI fluorescence indicates that LL-37 can kill C. albicans (Figure 4).
Figure 4. Candidacidal activity of LL-37. 1.5 µM PI (propidiumiodide) was added into C. albicans suspensions (106 cells/mL, 1 mL) and serially BL21-transformed bacteria supernatant (2 mL) mixture. The whole system was incubated at 30 ℃. During the 1 h incubation, the fluorescence was measured every 5 min at the excitation wavelength λexc 544 nm and emission wavelength λem 620 nm. The experiment was performed three times in triplicate. *, P < 0.05 from control using Student’s t test.
3. Conclusion
We successfully characterized LL-37 and demonstrated its ability to kill C. albicans from multiple angles.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 274
Illegal NgoMIV site found at 483
Illegal NgoMIV site found at 622 - 1000COMPATIBLE WITH RFC[1000]