Difference between revisions of "Part:BBa K3078004"
| Line 41: | Line 41: | ||
</p> | </p> | ||
</h5> | </h5> | ||
| − | [[File:B13-2.png| | + | [[File:B13-2.png|align left|B13-2]] |
<center> | <center> | ||
Figure 2. Expression of pVE-β-GA. Add 10 mg/ml Congo Red solution to LB medium containing β-1,3-glucan substrate (0.1 g/100 mL) at a ratio of 1:100. 100 μl supernatant obtained by centrifugation after ultrasonic crushing of E. coli with pVE-β-GA is added to the Oxford cup, and the pVE empty vector bacteria supernatant is used as the control. Stand at 37 ℃ for 24 hours. | Figure 2. Expression of pVE-β-GA. Add 10 mg/ml Congo Red solution to LB medium containing β-1,3-glucan substrate (0.1 g/100 mL) at a ratio of 1:100. 100 μl supernatant obtained by centrifugation after ultrasonic crushing of E. coli with pVE-β-GA is added to the Oxford cup, and the pVE empty vector bacteria supernatant is used as the control. Stand at 37 ℃ for 24 hours. | ||
Revision as of 02:58, 21 October 2019
β-1,3-glucanase
β-1,3-glucanase protein coding region. β-1,3-glucanase can degrade biofilm.
1. Usage and Biology
β-1,3-glucan is one of the primary components in C. albicans biofilm EPS, which is important for Candida biofilm formation and resistance to stresses. The enzyme β-1,3-glucanase, form Cellulosimicrobium cellulans, can degrade β-1,3-glucan. Therefore, this year, we decided use β-1,3-glucanase to disrupt the Candida biofilm matrix and increase the effect of the antimicrobial drug.
2. Characterization
We characterised β-1,3-glucanase by cloning it into pVE vector. Moreover, an signal peptide were added.
To verify the construction of pVE-β-1,3-glucanase(pVE-β-GA) which we generated, the digestion by SalI/EcoRV was performed by a standard protocol following agarose gel electrophoresis (Figure 1).
Figure 1. Digestion and agarose gel electrophoresis of pVE-β-GA.
To assess the expression of our β-1,3-glucanase, Congo Red experiment was used.
Congo Red has a strong chromogenic reaction with β-1, 3-glucan, and when β-1, 3-glucan is decomposed into reducing monosaccharides by β-1, 3-glucanase, the hydrolyzed region forms a pale yellow transparent hydrolytic circle. The results of activity detection of Congo Red hydrolytic ring (Figure 2) showed that compared to the control, there was a larger size of transparent hydrolytic circle caused by β-1, 3-glucanase expressed in E. coli with pVE-β-GA.
Figure 2. Expression of pVE-β-GA. Add 10 mg/ml Congo Red solution to LB medium containing β-1,3-glucan substrate (0.1 g/100 mL) at a ratio of 1:100. 100 μl supernatant obtained by centrifugation after ultrasonic crushing of E. coli with pVE-β-GA is added to the Oxford cup, and the pVE empty vector bacteria supernatant is used as the control. Stand at 37 ℃ for 24 hours.
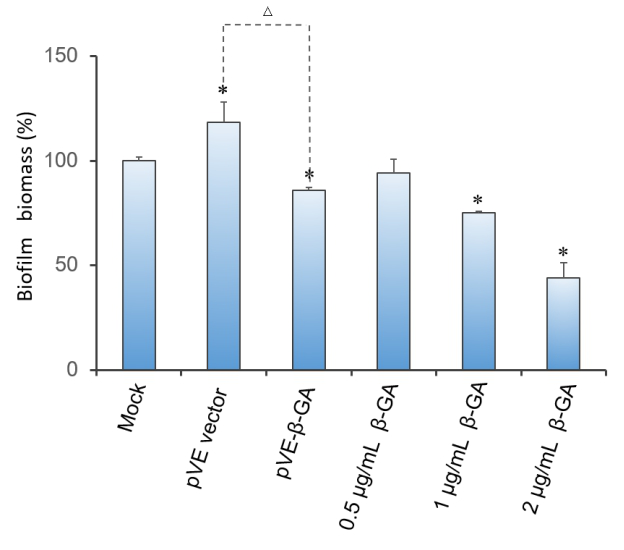
Crystal violet(CV) reduction method, which is commonly used for quantitative analysis of biofilm, was used to evaluate the antibiofilm activity of β-1,3-glucanase. The result indicates that β-1,3-glucanase has disruption effect on mature biofilm with concentration-dependent manner. Under the condition of using bacteria with pVE empty vector to exclude the influence of bacterial substances on the staining results, as Figure 3 shows, β-1,3-glucanase produced by our engineered bacteria has the effect of degrading biofilm. The supernatant of E. coli with pVE-β-GA diluted by one time is estimated to reach the effect of 0.5 μg/mL~2 μg/mL samples of standard β-1,3-glucanase.
Figure 3. Effect of β-1,3-glucanase on biofilm in 96-well microplate. Biofilm formed in RPMI 1640 medium for 48 h. Mature biofilm was treated with RPMI 1640 medium, supernatant of bacteria with pVE empty vector or pVE-β-GA and standard β-1,3-glucanase in different concentrations (0.5, 1 and 2 μg/mL) for another 24 h. Values obtained are given as the percentage of biofilm. The experiment was performed three times in triplicate. *, P < 0.05 from mock control using Student’s t test. △, P < 0.05.
3. Conclusion
Our engineered bacteria successfully characterized β-1,3-glucanase,moreover, it was effective in performing the function of degrading C. albicans biofilm.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 274
Illegal NgoMIV site found at 483
Illegal NgoMIV site found at 622 - 1000COMPATIBLE WITH RFC[1000]