Difference between revisions of "Part:BBa K864402"
| Line 15: | Line 15: | ||
<br> | <br> | ||
Summary: | Summary: | ||
| − | In this contribution we characterized the visual absorbance and the fluorescence of this construct. We also tested the oxygen dependency of the protein expression in <i>E. coli </i> BL21 (DE3) cells. | + | In this contribution we characterized the visual absorbance and the fluorescence of this construct. We also tested the oxygen dependency of the protein expression in <i>E. coli </i> BL21 (DE3) cells. The molecular weight of eforRed was also examined with a SDS-PAGE. |
Documentation: | Documentation: | ||
<html> | <html> | ||
| Line 25: | Line 25: | ||
</ul> | </ul> | ||
<br><br> | <br><br> | ||
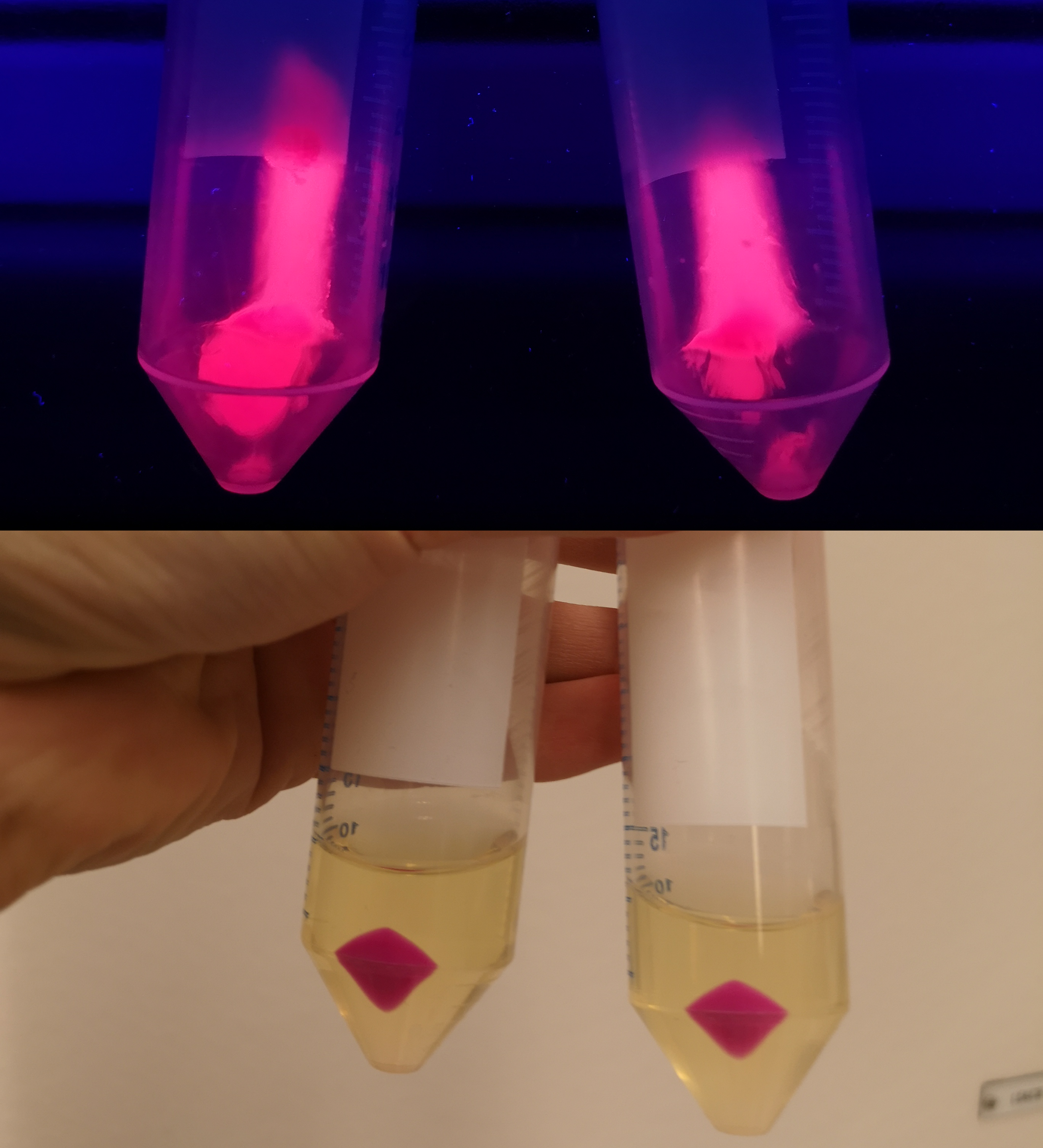
| − | <p> To verify | + | <p> To verify eforReds absorbance and emission, the construct was heat shocked into <i>Escherichia coli</i>, using the strain BL21 (DE3), and grown in a Falcon tube O.N. in 37 °C at 25 µg/mL chloramphenicol. Cotton plugs was used as corks for the Falcon tube. The bacterial solution was compared to a negative control with only BL21 (DE3)(Figure 1, left side) which showed the red color of eforRed in a bacterial solution. Thereafter, the eforRed expressing bacteria was centrifuged at 12 000 g for 10 minutes which displayed a burgundy colour (Figure 1, top-right corner). The pellet was also placed on an UV-table emitting light at 302 nm (Figure 1, down-right corner) and exhibited a pink glowing colour. These experiments verifies eforRed fluorescent effect as well as its absorbance in white light.</p> |
<br><br> | <br><br> | ||
</html>[[Image:T--Linkoping_Sweden--pcons-eforred.jpeg|460px]]<html> | </html>[[Image:T--Linkoping_Sweden--pcons-eforred.jpeg|460px]]<html> | ||
Revision as of 15:28, 20 October 2019
J23110-B0034-eforRed
eforRed eforRed is previously described as BBa_K592012. We submitted a functionally active variant with J23110 and B0034.
Contribution
Group: Linkoping_Sweden iGEM 2019
Author: Andreas Holmqvist and Leo Juhlin
Summary:
In this contribution we characterized the visual absorbance and the fluorescence of this construct. We also tested the oxygen dependency of the protein expression in E. coli BL21 (DE3) cells. The molecular weight of eforRed was also examined with a SDS-PAGE.
Documentation:
- the BBa_J23110Constitutive promotor
- the BBa_B0034Ribosome binding site
To verify eforReds absorbance and emission, the construct was heat shocked into Escherichia coli, using the strain BL21 (DE3), and grown in a Falcon tube O.N. in 37 °C at 25 µg/mL chloramphenicol. Cotton plugs was used as corks for the Falcon tube. The bacterial solution was compared to a negative control with only BL21 (DE3)(Figure 1, left side) which showed the red color of eforRed in a bacterial solution. Thereafter, the eforRed expressing bacteria was centrifuged at 12 000 g for 10 minutes which displayed a burgundy colour (Figure 1, top-right corner). The pellet was also placed on an UV-table emitting light at 302 nm (Figure 1, down-right corner) and exhibited a pink glowing colour. These experiments verifies eforRed fluorescent effect as well as its absorbance in white light.

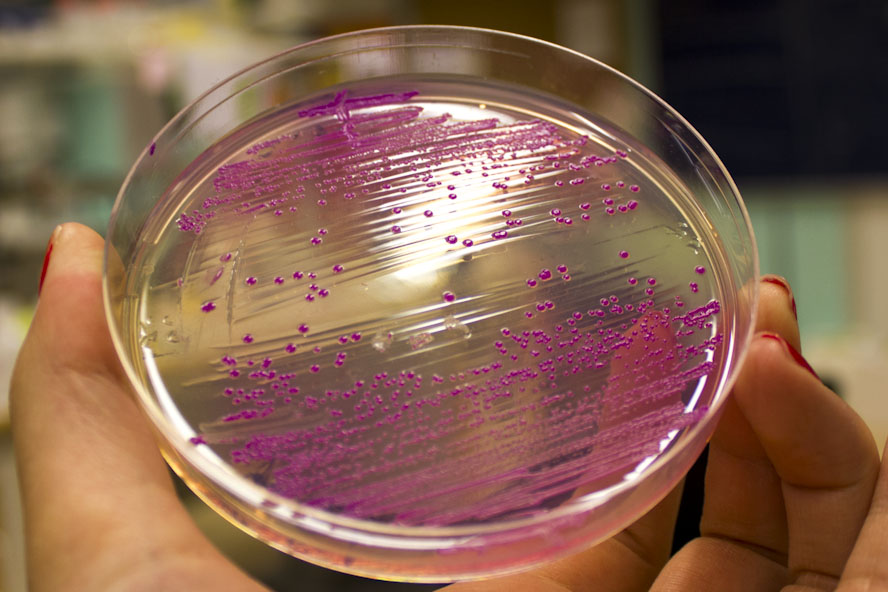
Further characterization was performed in order to demonstrate the absorbance and fluorescence of E. coli BL21 (DE3) containing pCons-eforRed. An eforRed culture was spread on an LB-agar plate containing 25 µg/ml chloramphenicol. The agar culture were photographed in visual light and on a UV-table emitting 302 nm (figure 2). The results were the same as above, in visual light (Figure 2, right) the cultures had a burgundy color and on the UV-table the bacteria exhibited a pink glowing colour (Figure 2, left).
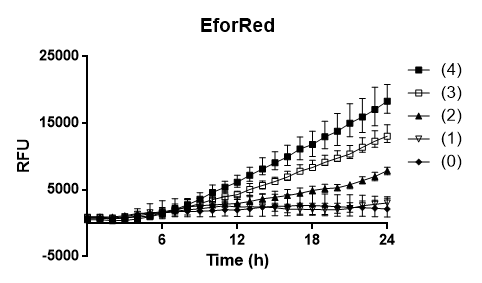
To test the oxygen dependency of the protein production of eforRed in E. coli BL21 (DE3) , a platereading was conducted. E. coli BL21 (DE3) was grown O.N. in 37 degrees Celsius to an 2.0 OD600 and diluted to 0.49 OD600, 200 ul of the the bacteria was pipetted into 96-well plates with replicates of 4. Oxygen access was varied by piercing different numbers of holes (0,1,2,3 and 4) in the plastic film of the 96-well plate. The experiment showed that 4 holes in plastic film gave the highest protein yield and that the access to oxygen effects E. colis BL21 (DE3) production of eforRed.
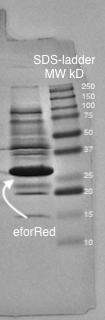
A study of the molecular weight of pCons eforRed expressed in E.coli BL21 (DE3) was done by sonicating the cells and performing a SDS-PAGE electrophoresis on the lysate (Figure 4.). Biorads "Precision Plus Protein Dual Color Standards" was used as the protein ladder in the electrophoresis.
Sequence and Features