Difference between revisions of "Part:BBa K2969028"
| Line 4: | Line 4: | ||
sfGFP-Asn39-Tyr151 is transformed from the reporting part sfGFP. The asparagine residue at 39 site and tyrosine residue at 151 site are both mutated to TAG codon. | sfGFP-Asn39-Tyr151 is transformed from the reporting part sfGFP. The asparagine residue at 39 site and tyrosine residue at 151 site are both mutated to TAG codon. | ||
| + | |||
| + | |||
| + | <h2>Characterization</h2> | ||
| + | |||
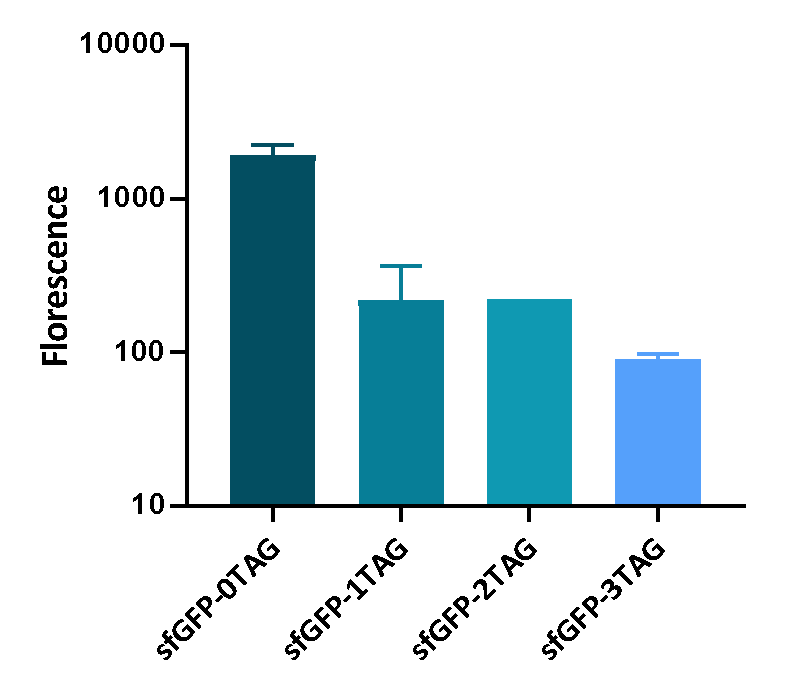
| + | <p>We inserted different number of TAG codon to sfGFP at different site to see the effect of TAG codon to the expression of sfGFP. Figure 1 shows that TAG codon can inhibit the transcription of sfGFP effectively. | ||
| + | </p> | ||
| + | <div>[[File:T--UCAS-China--sfGFP-nTAG.png|700px|thumb|center|<b>Figure 1:</b>The fluorescence of sfGEP inserted different number of TAG codon]] | ||
| + | </div> | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Latest revision as of 18:43, 19 October 2019
sfGFP-Asn39-Tyr151
sfGFP-Asn39-Tyr151 is transformed from the reporting part sfGFP. The asparagine residue at 39 site and tyrosine residue at 151 site are both mutated to TAG codon.
Characterization
We inserted different number of TAG codon to sfGFP at different site to see the effect of TAG codon to the expression of sfGFP. Figure 1 shows that TAG codon can inhibit the transcription of sfGFP effectively.
Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]