Difference between revisions of "Part:BBa K3128028"
(→Usage and Biology) |
|||
| Line 32: | Line 32: | ||
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
In 1989, Fields and Song demonstrated a new genetic system allowing the detection of protein-protein interaction <sup>(1)</sup>. At first, it was performed in ''Saccharomyces cerevisiae'' | In 1989, Fields and Song demonstrated a new genetic system allowing the detection of protein-protein interaction <sup>(1)</sup>. At first, it was performed in ''Saccharomyces cerevisiae'' | ||
| − | yeast and it was | + | yeast and it was named the yeast two-hybrid assay (Y2H). In 1998, Ladant and al. described the system in bacteria <sup>(2)</sup>. Nowadays, this biological technique is mostly |
| − | used to show and characterize the physical interaction between two cytosolic proteins or internal membrane proteins in vivo <sup>(3)</sup>. | + | used to show and characterize the physical interaction between two cytosolic proteins or internal membrane proteins <i>in vivo</i> <sup>(3)</sup>. |
<br> | <br> | ||
| Line 44: | Line 44: | ||
physiologically participates to the cellular transmission. <br> | physiologically participates to the cellular transmission. <br> | ||
| − | <br>This system involves the <i>Bordetella pertussis</i> '''adenylate cyclase'''which is the responsible agent for the pertussis disease. | + | <br>This system involves the <i>Bordetella pertussis</i> '''adenylate cyclase''' which is the responsible agent for the pertussis disease. |
Adenylate cyclase catalytic domain has the particularity to be splittable in two distinct parts: '''T18''' and '''T25''' sub-parts, <u>unable to fonction unless they | Adenylate cyclase catalytic domain has the particularity to be splittable in two distinct parts: '''T18''' and '''T25''' sub-parts, <u>unable to fonction unless they | ||
reassociate.</u> Each sub-part of the enzyme is fused with a protein of interest, either the bait or the prey protein chose beforehand by the experimentator. <br> | reassociate.</u> Each sub-part of the enzyme is fused with a protein of interest, either the bait or the prey protein chose beforehand by the experimentator. <br> | ||
| Line 69: | Line 69: | ||
<br>[http://2015.igem.org/Team:TU_Eindhoven Eindhoven-2015] iGEM project’s aim was to develop a “universal membrane sensor platform for biosensors”.<br> | <br>[http://2015.igem.org/Team:TU_Eindhoven Eindhoven-2015] iGEM project’s aim was to develop a “universal membrane sensor platform for biosensors”.<br> | ||
This year, '''Team Grenoble-Alpes''' is designing a new tears biosensor system based on [http://2015.igem.org/Team:TU_Eindhoven Eindhoven-2015]’s project. | This year, '''Team Grenoble-Alpes''' is designing a new tears biosensor system based on [http://2015.igem.org/Team:TU_Eindhoven Eindhoven-2015]’s project. | ||
| − | Both projects have a common base, the same receptors are used at the external surface of bacteria : '''[https://parts.igem.org/Part:BBa_K1492000 Clickable Outer Membrane Protein | + | Both projects have a common base, the same receptors are used at the external surface of bacteria : '''[https://parts.igem.org/Part:BBa_K1492000 Clickable Outer Membrane Protein X(COMP)]'''. <br> |
OmpX is an outer membrane protein with the C- and N-termini in the intracellular domain. To be able to use OmpX as a scaffold, a unnatural amino acid needs to be introduced. | OmpX is an outer membrane protein with the C- and N-termini in the intracellular domain. To be able to use OmpX as a scaffold, a unnatural amino acid needs to be introduced. | ||
This can be done by implementing the amber stop codon TAG in one of the loops of OmpX via a mutation. With a specific tRNA an azide-functionalized amino acid can be built in, | This can be done by implementing the amber stop codon TAG in one of the loops of OmpX via a mutation. With a specific tRNA an azide-functionalized amino acid can be built in, | ||
| − | which can be used for the SPAAC click chemistry reaction | + | which can be used for the SPAAC click chemistry reaction using DIBO functionalized groups, this modified protein is called '''COMP'''. |
| − | The complex '''aptamer''' fixed to a '''COMP''' is then | + | The complex '''aptamer''' fixed to a '''COMP''' is then named a '''COMB''' for '''Clickable Outer Membrane Biosensor'''.<br> |
<br>The Grenoble-Alpes team aims to develop an '''Outer membrane Bacterial Adenylate Cyclase Two Hybrid''' <i>(mBACTH)</i>.<br> | <br>The Grenoble-Alpes team aims to develop an '''Outer membrane Bacterial Adenylate Cyclase Two Hybrid''' <i>(mBACTH)</i>.<br> | ||
In this case, the two adenylate cyclase sub-parts are fused to the C-terminal ends of '''COMPs''' with a [https://parts.igem.org/wiki/index.php?title=Part:BBa_K3128010 Gly-Gly-Ser Linker (GGS)] of 54 amino acids <i>- in order to ensure a sufficient flexibility -</i>.<br> | In this case, the two adenylate cyclase sub-parts are fused to the C-terminal ends of '''COMPs''' with a [https://parts.igem.org/wiki/index.php?title=Part:BBa_K3128010 Gly-Gly-Ser Linker (GGS)] of 54 amino acids <i>- in order to ensure a sufficient flexibility -</i>.<br> | ||
| − | When ''' | + | When '''COMBs''' catch the extracellular target, they get closer, thus allowing the reconstitution of a functional adenylate cyclase due to the physical proximity of |
the two sub-parts.<br> | the two sub-parts.<br> | ||
| − | The enzyme is operational again and | + | The enzyme is operational again and produce a high quantity of '''cAMP''' <i>(around 17,000 mmol of cAMP formed per mg of adenylate cyclase per minute)</i>, |
| − | <u>the molecule responsible | + | <u>the molecule responsible for the signal transduction in the bacteria</u>.<br> |
https://static.igem.org/mediawiki/parts/e/ec/BACTH_1.gif <br> | https://static.igem.org/mediawiki/parts/e/ec/BACTH_1.gif <br> | ||
| − | <br>'''cAMP''' molecules diffuse to the cytoplasm of the bacterium and interact with catabolite activator proteins (CAP). | + | <br>'''cAMP''' molecules diffuse to the cytoplasm of the bacterium and interact with catabolite activator proteins (CAP) in a ratio 1 to 1. |
| − | + | Yhen two '''cAMP-CA'''P complexes are needed to activate the <u>expression of the gene under the control of the lactose promoter</u>.<br> | |
| − | Because of the high quantity of cAMP diffusing in the cytoplasm of the bacterium | + | Because of the high quantity of cAMP diffusing in the cytoplasm of the bacterium, the reporter gene is continously activated as long as cAMP is produced.<br> |
https://static.igem.org/mediawiki/parts/f/fd/BACTH_2.gif <br> | https://static.igem.org/mediawiki/parts/f/fd/BACTH_2.gif <br> | ||
| − | <br>The high enzymatic activity | + | <br>The high enzymatic activity of <i>Bordetella pertussis</i> Adenylate Cyclase generates a high production of '''cAMP''' in presence of ATP in the bacterium |
| − | thus <u>activating the | + | thus <u>activating the signalling cascade with the CAP-cAMP dependant promoter</u>.<br> |
| − | Hence this system is promising because it might have a great sensitivity and may | + | Hence this system is promising because it might have a great sensitivity and may drive a great signal amplification for a low amount of melocules to be detected.<br> |
</div> | </div> | ||
Revision as of 20:39, 17 October 2019
COMP fused with Leucine Zipper and T18 subpart of B.Pertussis AC under constitutive promoter
Contents
Sequence and features
This biobrick is composed of the external membrane Clickable Outer Membrane Protein X (COMP) protein fused at its C-terminal end with a 54 aa Gly-Gly-Ser Linker (GGS) followed by the T18 sub-part of the Bordetella Pertussis adenylate cyclase (AC) .
Leucine-zipper sequence is added between the signal peptide of OmpX -to express the recombiant protein in the external membrane- and COMP gene in order to force the physical closeness of Recombinants proteins.
COMP correspond to OmpX-WT which has a TAG codon (Y121F' mutation) which can be used to introduce any Non Natural Amino acid outside the cell (more informations). This biobrick constitutes one of the final part of the NeuroDrop device, because the non natural amino acid allows to present an aptamer to the external environment of the bacteria, and the intracellular part constitutes the signalling cascade of the biomarker detection (BACTH assay).
Leucine zippers are peptides which contain a hydrophobic leucine residue at every seventh position.
They dimerize through interactions between their helices.
Including a leucine zipper in the construct, between the signal peptide and the coding sequence of COMP is a powerful strategy to mimic the target biomarker recognition by the aptamers clicked on COMP recombinant protein expressed at the bacterial cell surface. Hence the adenylate cyclase activity will be restored through the interaction of both subparts and will induce the cAMP dependant signalling cascade.
This biobrick is under a constitutive promoter J23101, thereby the COMP fused with T18 protein is constitutively expressed leading to a large amount of recombinant protein expressed in the outer membrane at any time.
This BioBrick intended to operate with BBa_K3128029.
These two biobricks are the positive condition of the mBACTH -Leucine Zipper condition-.
OmpX proteins are fused to the adenylate cyclase sub-parts at their C-terminal moiety and to Leucine Zipper at their C-teminal part.
Thereby they are forced to get closer to form dimers in bacterial external membrane leading to the reconstitution of the adenylate cyclase and mimic the behaviour proteins would have under extracellular target biomolecule recognition.
The signal measured is considered as the positive control of our system.
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1436
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 977
Illegal NgoMIV site found at 1387
Illegal AgeI site found at 1193 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 179
Usage and Biology
In 1989, Fields and Song demonstrated a new genetic system allowing the detection of protein-protein interaction (1). At first, it was performed in Saccharomyces cerevisiae
yeast and it was named the yeast two-hybrid assay (Y2H). In 1998, Ladant and al. described the system in bacteria (2). Nowadays, this biological technique is mostly
used to show and characterize the physical interaction between two cytosolic proteins or internal membrane proteins in vivo (3).
Bacterial Adenylate Cyclase Two-Hybrid (BACTH)
The principle lies on the interaction-mediated reconstitution of a signalling cascade in Escherichia coli. The messenger molecule involved in this cascade is the
cyclic adenosine monophosphate (cAMP) produced by the adenylate cyclase. Adenylate cyclase is an enzyme catalysing the cAMP production from ATP. It
physiologically participates to the cellular transmission.
This system involves the Bordetella pertussis adenylate cyclase which is the responsible agent for the pertussis disease.
Adenylate cyclase catalytic domain has the particularity to be splittable in two distinct parts: T18 and T25 sub-parts, unable to fonction unless they
reassociate. Each sub-part of the enzyme is fused with a protein of interest, either the bait or the prey protein chose beforehand by the experimentator.
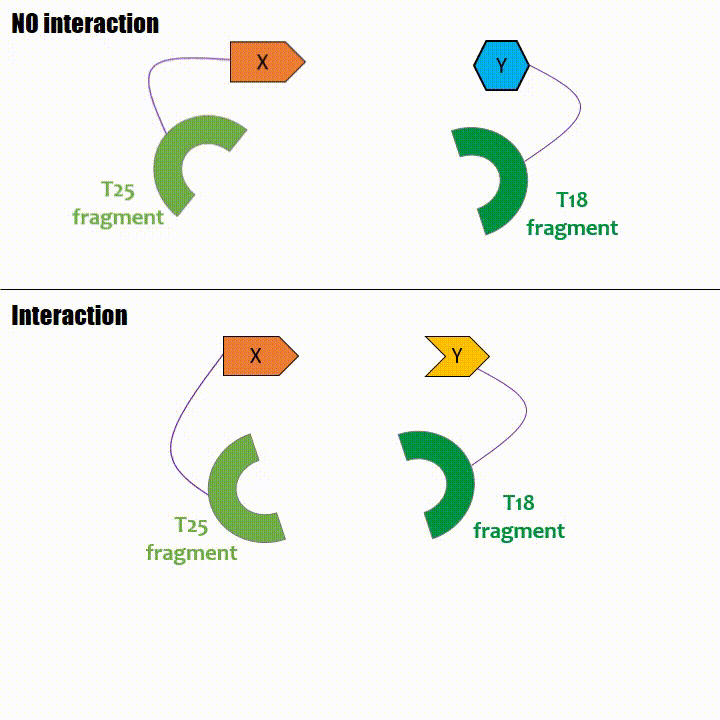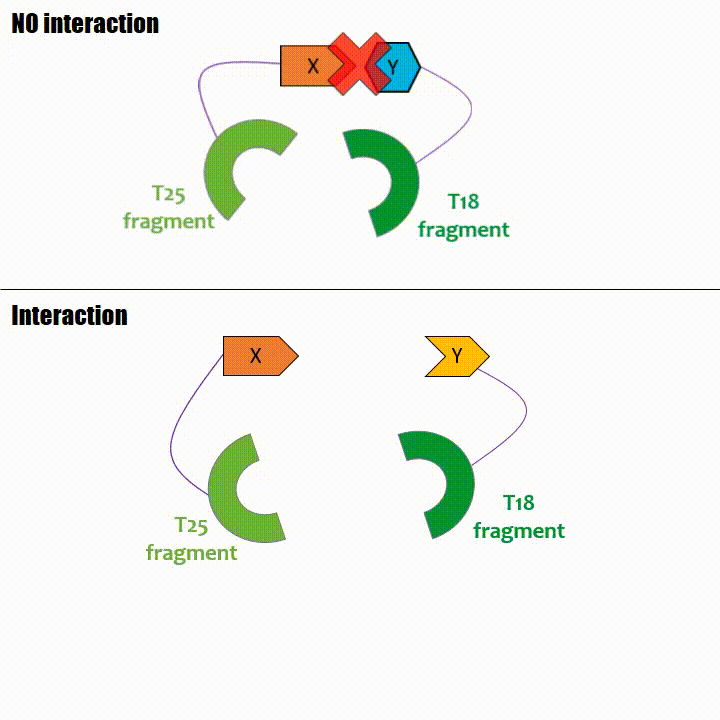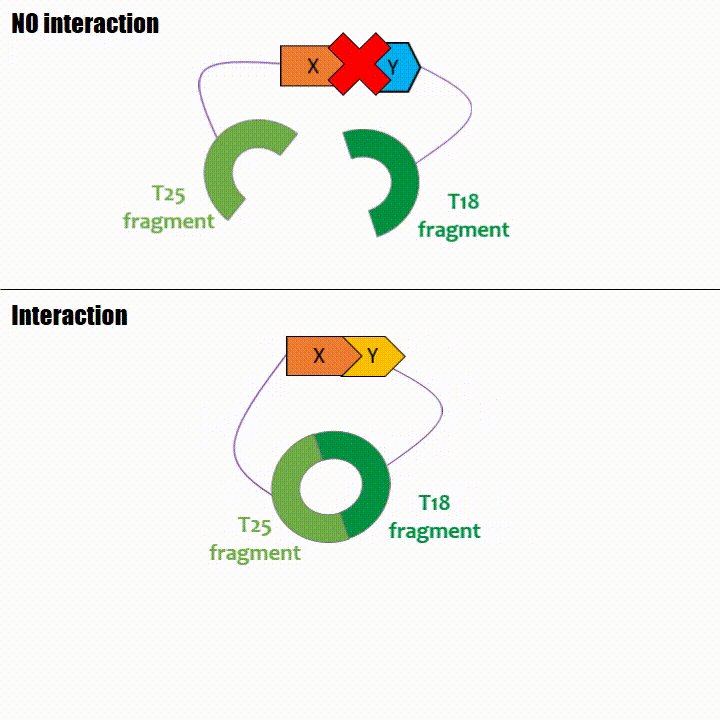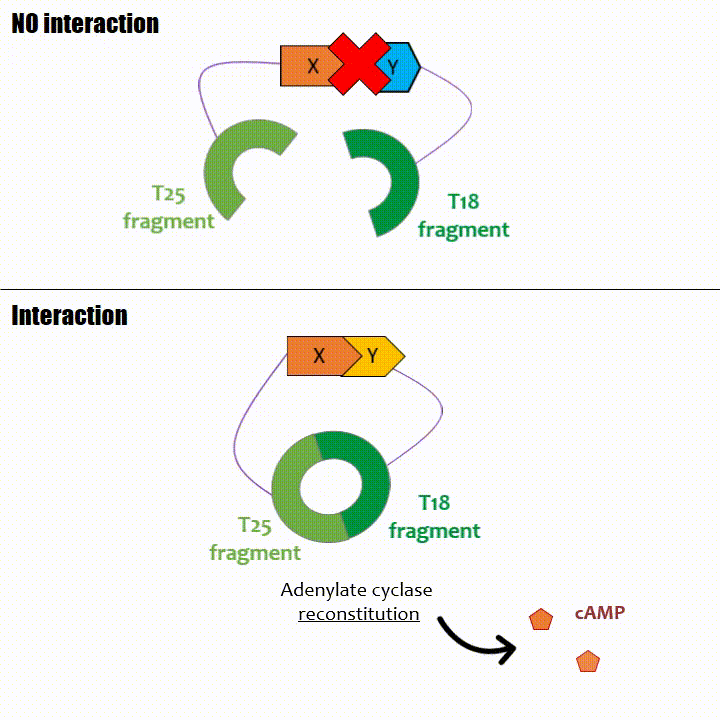
If two proteins interact, then T18 and T25 are bring together and reconstitute a functional adenylate cyclase enzyme thus enabling cAMP production. Using
cya- bacteria – strain for whom the adenylate gene is deleted, involving an absence of this endogenous enzyme – a BACTH could be done with the creation of two
fusion proteins : the first one, fused at its N or C terminal intracellular end with the T18 sub-part; the second one fused with the T25 sub-part.
The interaction of these proteins of interest will lead to the adenylate cyclase reconstitution, thus initiating cAMP production. The cAMP produced will act as a
messenger by fixing itself to the transcriptional activator CAP, cAMP form the CAP-cAMP complex, controlling the expression of the lactose promoter by initiating transcription of the following gene.
This promoter is placed upstream the chosen reporter gene.
NeuroDrop Project - Outer-Membrane BACTH (mBACTH)

[http://2015.igem.org/Team:TU_Eindhoven Eindhoven-2015] iGEM project’s aim was to develop a “universal membrane sensor platform for biosensors”.
This year, Team Grenoble-Alpes is designing a new tears biosensor system based on [http://2015.igem.org/Team:TU_Eindhoven Eindhoven-2015]’s project.
Both projects have a common base, the same receptors are used at the external surface of bacteria : Clickable Outer Membrane Protein X(COMP).
OmpX is an outer membrane protein with the C- and N-termini in the intracellular domain. To be able to use OmpX as a scaffold, a unnatural amino acid needs to be introduced.
This can be done by implementing the amber stop codon TAG in one of the loops of OmpX via a mutation. With a specific tRNA an azide-functionalized amino acid can be built in,
which can be used for the SPAAC click chemistry reaction using DIBO functionalized groups, this modified protein is called COMP.
The complex aptamer fixed to a COMP is then named a COMB for Clickable Outer Membrane Biosensor.
The Grenoble-Alpes team aims to develop an Outer membrane Bacterial Adenylate Cyclase Two Hybrid (mBACTH).
In this case, the two adenylate cyclase sub-parts are fused to the C-terminal ends of COMPs with a Gly-Gly-Ser Linker (GGS) of 54 amino acids - in order to ensure a sufficient flexibility -.
When COMBs catch the extracellular target, they get closer, thus allowing the reconstitution of a functional adenylate cyclase due to the physical proximity of
the two sub-parts.
The enzyme is operational again and produce a high quantity of cAMP (around 17,000 mmol of cAMP formed per mg of adenylate cyclase per minute),
the molecule responsible for the signal transduction in the bacteria.

cAMP molecules diffuse to the cytoplasm of the bacterium and interact with catabolite activator proteins (CAP) in a ratio 1 to 1.
Yhen two cAMP-CAP complexes are needed to activate the expression of the gene under the control of the lactose promoter.
Because of the high quantity of cAMP diffusing in the cytoplasm of the bacterium, the reporter gene is continously activated as long as cAMP is produced.

The high enzymatic activity of Bordetella pertussis Adenylate Cyclase generates a high production of cAMP in presence of ATP in the bacterium
thus activating the signalling cascade with the CAP-cAMP dependant promoter.
Hence this system is promising because it might have a great sensitivity and may drive a great signal amplification for a low amount of melocules to be detected.
References
(1) Fields S, Song O. A novel genetic system to detect protein–protein interactions. Nature [Internet]. 1989
(2) Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. PNAS [Internet]. 1998
(3) Karimova G, Gauliard E, Davi M, P.Ouellette S, Ladant D. Protein–Protein Interaction: Bacterial Two-Hybrid. 2017
Fields S, Song O. A novel genetic system to detect protein–protein interactions. Nature. (1989)
Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. PNAS. (1998)
Karimova G, Gauliard E, Davi M, P.Ouellette S, Ladant D. Protein–Protein Interaction: Bacterial Two-Hybrid. (2017)
Picture of the reaction ATP-cAMP. Khan Academy Website. Retrieved October 10, 2019, from https://www.khanacademy.org
Euromedex, BACTH System Kit available here
Leusch, Paulaitis, Friedman. Adenylate cyclase toxin of Bordetella pertussis: production, purification, and partial characterization. Am Soc Microbiol | Infect Immun. (1990)
Hantke, Winkler, Schultz. Escherichia coli exports cyclic AMP via TolC. J Bacteriol. (2011)
Eindhoven 2015 Website, from http://2015.igem.org/Team:TU_Eindhoven/Project/Design
