Difference between revisions of "Part:BBa K3245011"
| Line 1: | Line 1: | ||
| + | <h1>BBa_K3245011:</h1> | ||
| + | <p>BBa_K3245011(J23112-B0034-C0040-B0015-R0040-B0034-K3245008-B0014-B0015) is one of a series of composite parts designed for characterizing R0040. </p> | ||
| + | <h2>Usage and biology:</h2> | ||
| + | <p>This part is one of a collection of parts designed for characterizing ptetR (R0040). This part constantly expresses tetR(C0040) at low level strength to inhibit the downstream ptetR from expressing sfGFP. When induced by aTc/tet, this part shows a leaping induction curve. To use this part, simply clone it into a middle/high copy plasmid vector, transfer into E.coli K-12, incubate overnight, and induce with aTc after proper dilution. </p> | ||
| + | <h2>Design:</h2> | ||
| + | <p>Since our project involves tetR-ptetR double plasmid expression system (Fig.1), it is essential for us to characterize ptetR by measuring the level of tetR expression required for total inhibition. To achieve this, we designed 3 different tetR-ptetR expression systems (BBa_K3245003, BBa_K3245012, BBa_K3245011), among which K3245011 expresses the lowest amount of tetR (J23106-B0034). </p> | ||
| + | <p>In order not to let the promoter of antibiotics and J23112 affect the process of characterization, we inserted 2 B0015s into our expression system, one at the upstream of R0040, and the other at the downstream of sfGFP. </p> | ||
| + | [[File:T--Fudan--Part7.png|700px]] | ||
| + | |||
| + | <h2>Characterization:</h2> | ||
| + | <p>In order to characterize R0040, we cloned K3245011 into p15A, a vector with middle copy number (15-20). We then transferred p15A-K3245011 into E.coli DH10B and incubated it overnight. The culture was diluted with LB to 1/500 before induction (see team Fudan_protocol for more detail). Due to lack of access to aTc, we use tetracycline (tet) to induce R0040. Our results are shown as follows: </p> | ||
| + | [[File:T--Fudan--Part8.jpg|600px]] | ||
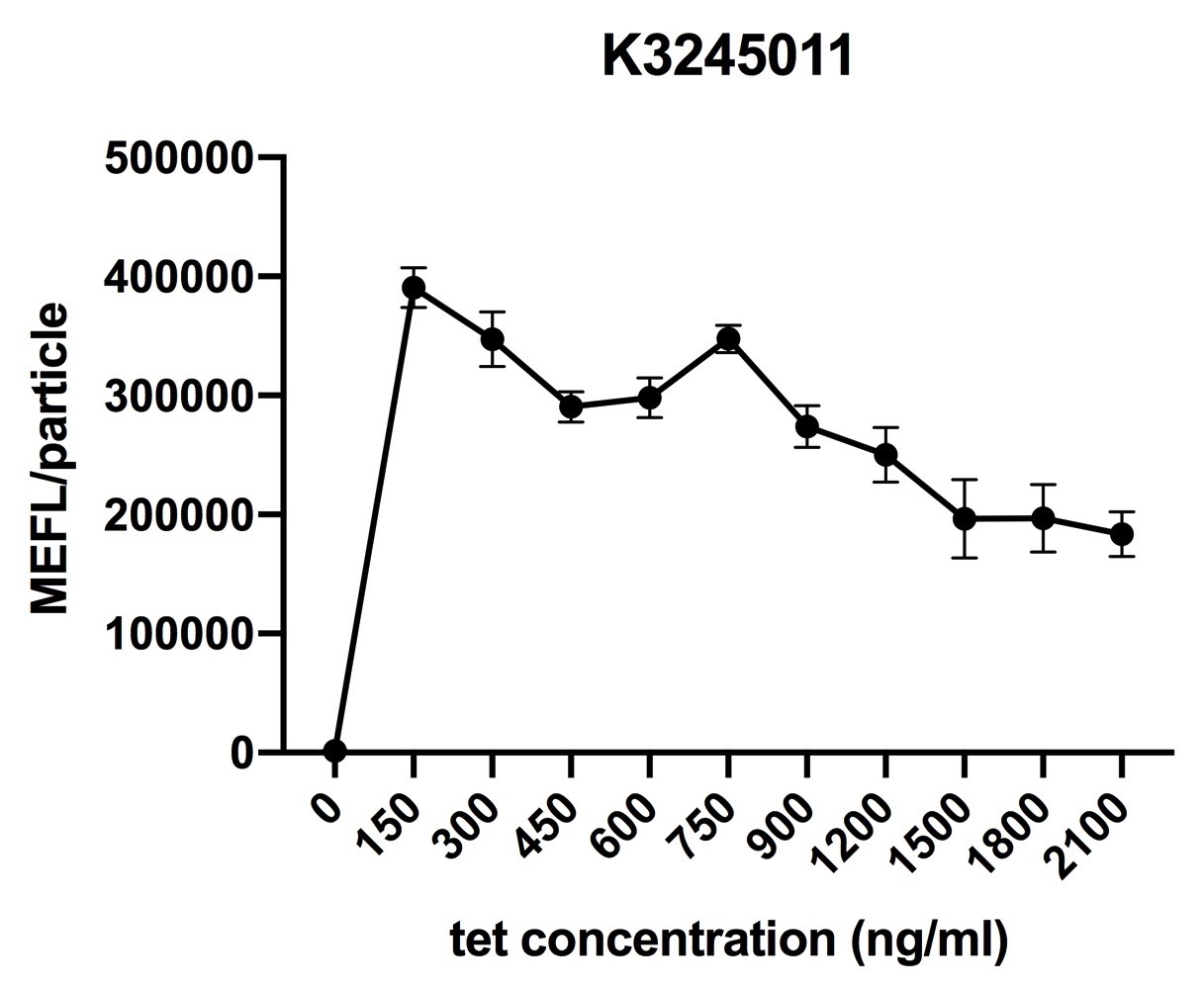
| + | <p>Fig.1 induction curve of K3245011 (7 hours). Data are collected and analyzed according to iGEM standard data analysis form after 7 hours of induction. </p> | ||
| + | [[File:T--Fudan--Part9.jpg|700px]] | ||
| + | <p>Fig.2 figure showing different induction curves of K3245011 under different tet concentration with the lapse of time. Data are collected and analyzed according to iGEM standard data analysis form after 7 hours of induction.</p> | ||
| + | |||
| + | |||
__NOTOC__ | __NOTOC__ | ||
Latest revision as of 16:17, 11 October 2019
BBa_K3245011:
BBa_K3245011(J23112-B0034-C0040-B0015-R0040-B0034-K3245008-B0014-B0015) is one of a series of composite parts designed for characterizing R0040.
Usage and biology:
This part is one of a collection of parts designed for characterizing ptetR (R0040). This part constantly expresses tetR(C0040) at low level strength to inhibit the downstream ptetR from expressing sfGFP. When induced by aTc/tet, this part shows a leaping induction curve. To use this part, simply clone it into a middle/high copy plasmid vector, transfer into E.coli K-12, incubate overnight, and induce with aTc after proper dilution.
Design:
Since our project involves tetR-ptetR double plasmid expression system (Fig.1), it is essential for us to characterize ptetR by measuring the level of tetR expression required for total inhibition. To achieve this, we designed 3 different tetR-ptetR expression systems (BBa_K3245003, BBa_K3245012, BBa_K3245011), among which K3245011 expresses the lowest amount of tetR (J23106-B0034).
In order not to let the promoter of antibiotics and J23112 affect the process of characterization, we inserted 2 B0015s into our expression system, one at the upstream of R0040, and the other at the downstream of sfGFP.
Characterization:
In order to characterize R0040, we cloned K3245011 into p15A, a vector with middle copy number (15-20). We then transferred p15A-K3245011 into E.coli DH10B and incubated it overnight. The culture was diluted with LB to 1/500 before induction (see team Fudan_protocol for more detail). Due to lack of access to aTc, we use tetracycline (tet) to induce R0040. Our results are shown as follows:
Fig.1 induction curve of K3245011 (7 hours). Data are collected and analyzed according to iGEM standard data analysis form after 7 hours of induction.
Fig.2 figure showing different induction curves of K3245011 under different tet concentration with the lapse of time. Data are collected and analyzed according to iGEM standard data analysis form after 7 hours of induction.
Part constructed to characterize part R0040.
As we use tetR-ptetR system in our project, it is essential for us to measure the minimum amount of tetR needed to stop ptetR. Considering that ptetR is a rather strict regulatory promoter, we constructed 4 tetR-ptetR-GFP expression systems,each with different strength of tetR. Then we measured both their initial GFP strength as well as their induction curve
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]