Difference between revisions of "Part:BBa K3245009"
| Line 1: | Line 1: | ||
| + | <p>Part Name luxpR-HS100</p> | ||
| + | <h2>Description:</h2> | ||
| + | <p>This hybrid promoter has lower expression level when induced by luxR-AHL complex triggered by quorum sensing ( QS ) in some Gram-negative bacteria, meanwhile it’s leakage is also lower when not induced. </p> | ||
| + | <h2>Usage and biology:</h2> | ||
| + | <p>This fused promoter is designed for the situation that QS effect and low leakage are required at the same time in a circuit if a little expression loss is acceptable to some extent. We applied this improved promoter to link upstream QS regulation with tetR suppression effect downstream. It’s a successful trial since tetR-ptetR system is rather strict and luxpR ( WT ) is unsuitable due to its high leakage.</p> | ||
| + | <h2>Design:</h2> | ||
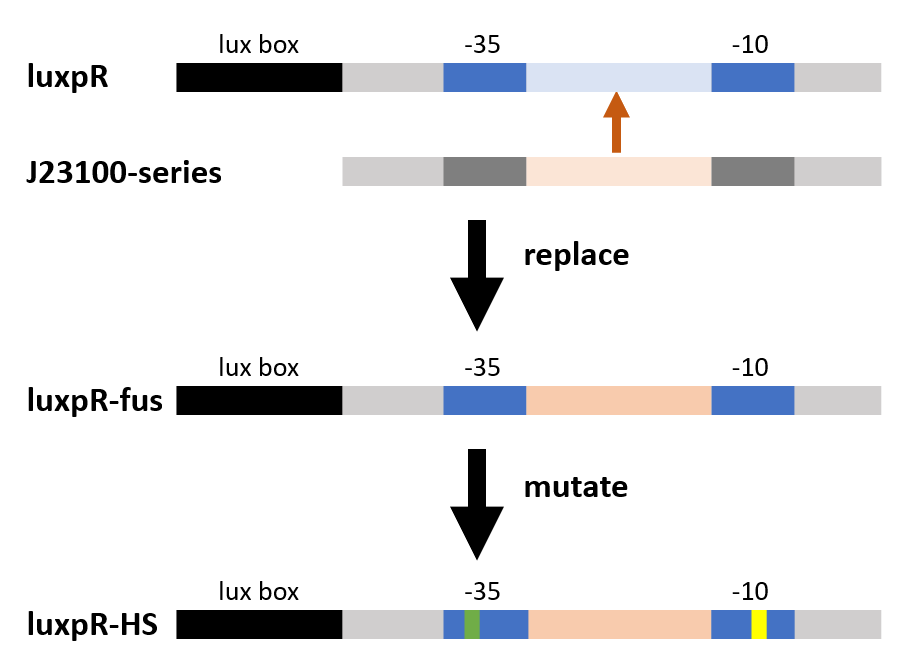
| + | [[File:T--Fudan--PartT3.png|700px]] | ||
| + | <p>As the figure shows, we substituted the -35 to -10 region of the original promoter luxpR with the one of J23100, a strong constitutive promoter. This region is rarely concerned as it’s rather conservative in promoters with the same function, but it has crucial structural effect on σ factor binding and other events in transcription regulation. Fortunately the change proved to be effective on adjusting the behavior of the regulatory promoter.</p> | ||
| + | |||
| + | <h2>Characterization:</h2> | ||
| + | |||
| + | <p>1. Transform a control plasmid containing luxI and luxR ( all from official parts or BBa_K3245002 ) with pUC ori ( high copy number ) and an effect plasmid containing luxpR-HS100 and GFP behind the promoter ( BBa_K3245015 ) with p15A ori (medium copy number ) into DH10B. </p> | ||
| + | <p>2. Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 3 ml LB with ampicillin at 100 μg/ml. Incubate overnight at 37℃ in a shaker.</p> | ||
| + | <p>3. Measure and keep all groups OD600 reach 1. Inocuate each group with with 1/5000 concentration. Incubate 12 hours at 37℃ in a shaker.</p> | ||
| + | <p>4. Add 100 µl bacteria culture medium into each well of a 96-well plate. One well of LB as blank, one group of wild type DH10B as control.</p> | ||
| + | <p>5. Measure OD600 and fluorescence continuously every 30 minutes with a microplate reader.</p> | ||
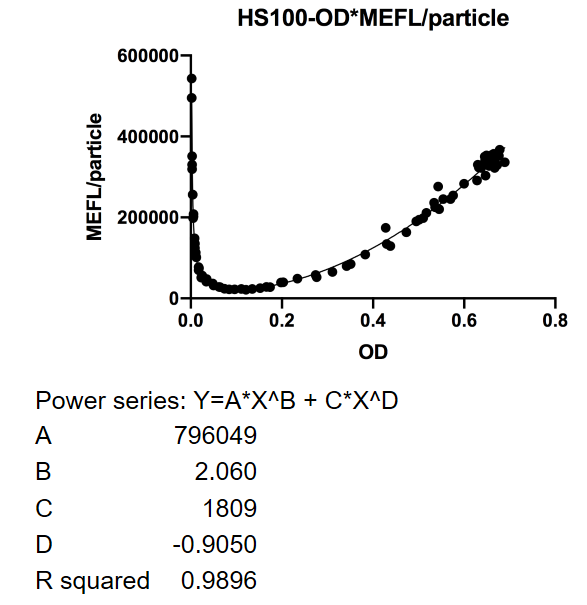
| + | [[File:T--Fudan--PartBBaK32450092.png|400px]] | ||
| + | <p>This figure used OD600 as X-axis unit. The downward curve before OD600 <0.1 is due to the parental fluorescence and the dilution effect after proliferation. Data points are shown in the figure for fitting analysis.</p> | ||
| + | <h2>Reference</h2> | ||
| + | <p>Antunes, L. C., et al. "A mutational analysis defines Vibrio fischeri LuxR binding sites." Journal of Bacteriology 190.13(2008):4392-4397. </p> | ||
| + | <p>de Boer HA, Comstock LJ, Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983;80(1):21–25. doi:10.1073/pnas.80.1.21</p> | ||
Revision as of 11:57, 20 October 2019
Part Name luxpR-HS100
Description:
This hybrid promoter has lower expression level when induced by luxR-AHL complex triggered by quorum sensing ( QS ) in some Gram-negative bacteria, meanwhile it’s leakage is also lower when not induced.
Usage and biology:
This fused promoter is designed for the situation that QS effect and low leakage are required at the same time in a circuit if a little expression loss is acceptable to some extent. We applied this improved promoter to link upstream QS regulation with tetR suppression effect downstream. It’s a successful trial since tetR-ptetR system is rather strict and luxpR ( WT ) is unsuitable due to its high leakage.
Design:
As the figure shows, we substituted the -35 to -10 region of the original promoter luxpR with the one of J23100, a strong constitutive promoter. This region is rarely concerned as it’s rather conservative in promoters with the same function, but it has crucial structural effect on σ factor binding and other events in transcription regulation. Fortunately the change proved to be effective on adjusting the behavior of the regulatory promoter.
Characterization:
1. Transform a control plasmid containing luxI and luxR ( all from official parts or BBa_K3245002 ) with pUC ori ( high copy number ) and an effect plasmid containing luxpR-HS100 and GFP behind the promoter ( BBa_K3245015 ) with p15A ori (medium copy number ) into DH10B.
2. Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 3 ml LB with ampicillin at 100 μg/ml. Incubate overnight at 37℃ in a shaker.
3. Measure and keep all groups OD600 reach 1. Inocuate each group with with 1/5000 concentration. Incubate 12 hours at 37℃ in a shaker.
4. Add 100 µl bacteria culture medium into each well of a 96-well plate. One well of LB as blank, one group of wild type DH10B as control.
5. Measure OD600 and fluorescence continuously every 30 minutes with a microplate reader.
This figure used OD600 as X-axis unit. The downward curve before OD600 <0.1 is due to the parental fluorescence and the dilution effect after proliferation. Data points are shown in the figure for fitting analysis.
Reference
Antunes, L. C., et al. "A mutational analysis defines Vibrio fischeri LuxR binding sites." Journal of Bacteriology 190.13(2008):4392-4397.
de Boer HA, Comstock LJ, Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983;80(1):21–25. doi:10.1073/pnas.80.1.21