Difference between revisions of "Part:BBa K592100"
(→Usage and Biology) |
|||
| Line 25: | Line 25: | ||
[http://www.ncbi.nlm.nih.gov/pubmed/18940671] Subach, O. M., I. S. Gundorov, et al. (2008). "Conversion of red fluorescent protein into a bright blue probe." Chem Biol 15(10): 1116-24. | [http://www.ncbi.nlm.nih.gov/pubmed/18940671] Subach, O. M., I. S. Gundorov, et al. (2008). "Conversion of red fluorescent protein into a bright blue probe." Chem Biol 15(10): 1116-24. | ||
| + | |||
| + | ===iangsu_High_School 2019’s Characterisation=== | ||
| + | |||
| + | ===BBa_K592100 Blue Fluorescent Protein (mTagBFP)=== | ||
| + | <p>The E. coli expressing blue fluorescent protein(mTagBFP) were cultured at 28 °C and the overnight bacterial solution was collected for subsequent measurement.</p> | ||
| + | <p>After ultrasonically disruption of the above bacterial solution ,5 ul of each was added to 4 kinds of 45 ul solution having a pH of 3, 5, 7, and 9, and the fluorescence was measured using varioskan flash of Thermo Scientific. The excitation wavelength/emission wavelength corresponding to each fluorescent protein is: BFP:402/457.</p> | ||
| + | |||
| + | |||
| + | <p>Figure Fluorescence of BFP under different pH. There were two kinds of BFP expressing in E.coli(BFP) and expressing in E.coli after ultrasonically disruption(BFP-UC). Under the same pH, the fluorescence of BFP was higher than BFP-UC. With increasing of pH( among pH3, pH5, pH7,), the fluorescence of BFP and BFP-UC all raised. When the pH increased from pH7 to pH9, the fluorescence of BFP and BFP-UC were all down. </p> | ||
| + | |||
| + | <p>The results showed that the fluorescent value of each fluorescent protein did not change significantly in acid and alkali circumstance before sonication, but after sonication, each fluorescent protein showed the highest fluorescent value at pH 7, and the fluorescent value decreased as the environment became more acidic.</p> | ||
<!-- --> | <!-- --> | ||
Revision as of 08:56, 17 October 2019
Blue Fluorescent Protein (mTagBFP)
This part codes for the bright blue fluorescent protein mTagBFP. mTagBFP is a monomeric protein with a narrow fluorescence emission spectrum with a maximum at 456 nm. It has a tyrosine-based chromophore, giving it substantially higher brightness, faster chromophore maturation and higher pH stability than blue fluorescent proteins with a histidine in the chromophore. Subach et. al. started with a red fluorescent protein (TagRFP) and converted it to a bright blue monomeric protein. See the article listed in source. The sequence has been codon optimized for expression in E coli by DNA 2.0.
Subach et. al. reported the following data:
Excitation peak: 399 nm. Emission peak: 456 nm. Maturation Half-Time (min): 13. Relative brightness to EBFP2: 1.82. Extinction Coefficient (M-1 cm-1): 52000. Quantum Yield: 0.63.
Usage and Biology
This part is useful as a reporter.
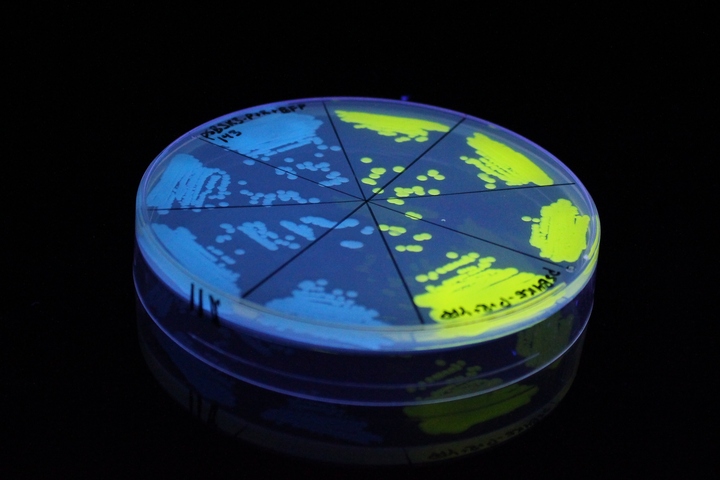
iGEM11_Uppsala-Sweden. Fluorescence on UV table. The images above show E coli constitutively expressing mTagBFP BBa_K592100 (blue) and YFP BBa_K592101 (yellow) illuminated on a UV table.
References
[http://www.ncbi.nlm.nih.gov/pubmed/18940671] Subach, O. M., I. S. Gundorov, et al. (2008). "Conversion of red fluorescent protein into a bright blue probe." Chem Biol 15(10): 1116-24.
iangsu_High_School 2019’s Characterisation
BBa_K592100 Blue Fluorescent Protein (mTagBFP)
The E. coli expressing blue fluorescent protein(mTagBFP) were cultured at 28 °C and the overnight bacterial solution was collected for subsequent measurement.
After ultrasonically disruption of the above bacterial solution ,5 ul of each was added to 4 kinds of 45 ul solution having a pH of 3, 5, 7, and 9, and the fluorescence was measured using varioskan flash of Thermo Scientific. The excitation wavelength/emission wavelength corresponding to each fluorescent protein is: BFP:402/457.
Figure Fluorescence of BFP under different pH. There were two kinds of BFP expressing in E.coli(BFP) and expressing in E.coli after ultrasonically disruption(BFP-UC). Under the same pH, the fluorescence of BFP was higher than BFP-UC. With increasing of pH( among pH3, pH5, pH7,), the fluorescence of BFP and BFP-UC all raised. When the pH increased from pH7 to pH9, the fluorescence of BFP and BFP-UC were all down.
The results showed that the fluorescent value of each fluorescent protein did not change significantly in acid and alkali circumstance before sonication, but after sonication, each fluorescent protein showed the highest fluorescent value at pH 7, and the fluorescent value decreased as the environment became more acidic.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]