Difference between revisions of "Part:BBa K2728001"
Bluepumpkin (Talk | contribs) |
Bluepumpkin (Talk | contribs) (→Experimental Characterization) |
||
| Line 24: | Line 24: | ||
<br /> | <br /> | ||
=== Experimental Characterization === | === Experimental Characterization === | ||
| + | Though we failed a lot of times that we cannot even count, due to a lot of different reasons and controls. Eventually we found the proper setting of our equipment, and got some promising data as below: | ||
| + | <br /><br /> | ||
| + | ===== Experiment 1 ===== | ||
| + | Culture environment: saturated formaldehyde aqueous solution with concentration of 37 percent.<br /> | ||
| + | Instruments: tecan infinite m1000<br /> | ||
| + | Experiment group: BL21 with pFrmR+GFP<br /> | ||
| + | Control group: BL21 (with no plasmid transformed)<br /> | ||
<br /> | <br /> | ||
| + | #Test the OD number of overnight-cultured strain; | ||
| + | #Diluted to 0.05, the strains are cultured under 37 celsius, 220prm. | ||
| + | #Take 5 tubes of 1ml system both from experiment group and control group and add 0ul, 1ul, 2.5ul, 5ul, and 7.5ul saturated formaldehyde solution into each tube. Mix evenly. | ||
| + | #ake 150ul from each of the tubes into 96 orifice plate. | ||
| + | #est fluorescence in tecan infinite m1000. | ||
<br /> | <br /> | ||
| + | Related conditions:<br /> | ||
| + | Excitation Wavelength 495 nm<br /> | ||
| + | Emission Wavelength 525 nm<br /> | ||
| + | List of actions in this measurement script:<br /> | ||
| + | Shaking (Orbital) Duration: 900 s<br /> | ||
| + | Shaking (Orbital) Amplitude: 2 mm<br /> | ||
| + | Shaking (Orbital) Frequency: 306 rpm<br /> | ||
| + | <br /> | ||
| + | Results:<br /> | ||
| + | [[File:T--BGIC-Global--pfmxrxp1.png|left|border|800px]]<br clear=all> | ||
| + | |||
| + | <br /> | ||
| + | <br /> | ||
| + | |||
=== Improvements === | === Improvements === | ||
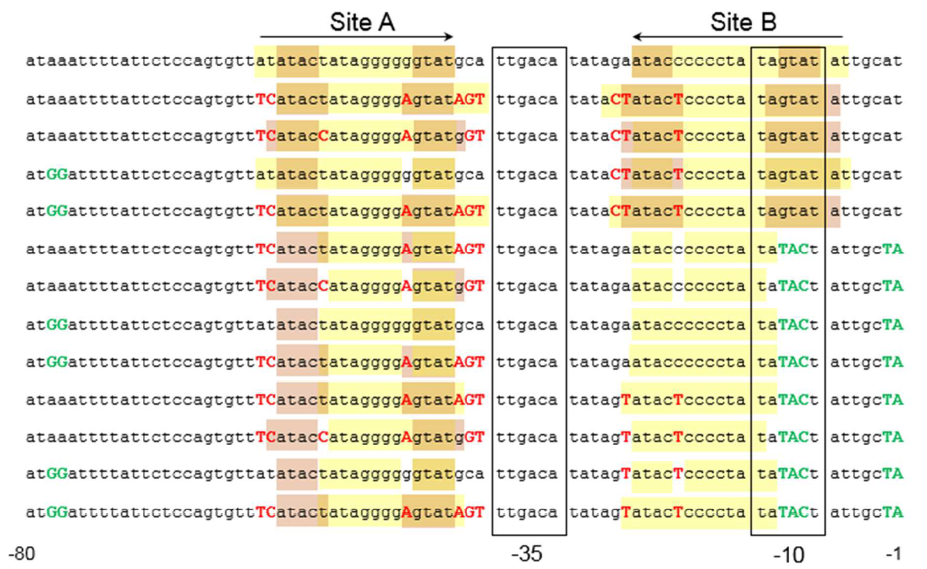
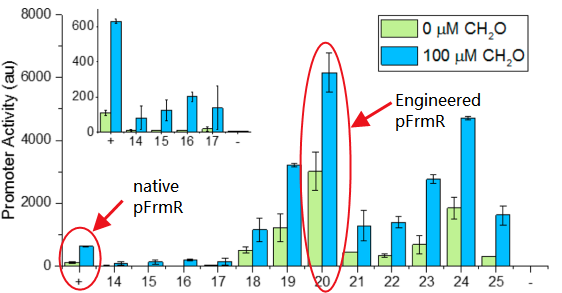
The sequence of this part was taken from the research of Rohlhill J. et al. The 2 binding sites of variations are -35 and -10 (Fig 1). We ordered synthesized plasmid with pFrmR and EGFP from Gensceipt and constructed pFrmR-EGFP-FrmR reporter system on plasmid pUC57. After dosing formaldehyde of 100 to 400uM, we tested the EGFP expression to identify the activity of the formaldehyde induced response of this prompter. | The sequence of this part was taken from the research of Rohlhill J. et al. The 2 binding sites of variations are -35 and -10 (Fig 1). We ordered synthesized plasmid with pFrmR and EGFP from Gensceipt and constructed pFrmR-EGFP-FrmR reporter system on plasmid pUC57. After dosing formaldehyde of 100 to 400uM, we tested the EGFP expression to identify the activity of the formaldehyde induced response of this prompter. | ||
Revision as of 16:42, 17 October 2018
pfrmR - An Engineered Formaldehyde-Inducible Promoter
Basic Description
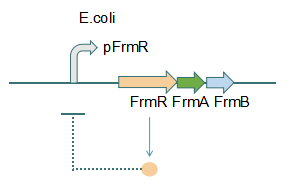
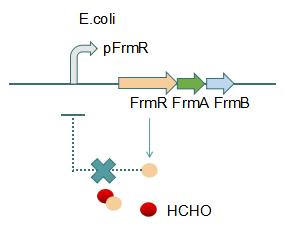
This promoter is an engineered formaldehyde-inducible promoter. Escherichia coli has a native formaldehyde-inducible promoter, pfrm, which is found upstream of the frmRAB formaldehyde detoxification operon. FrmR, the first product of the operon, is a member of the DUF156 family of DNA-binding transcriptional regulators. It binds the frmRAB promoter region and is negatively allosterically modulated by formaldehyde. FrmR is specific to formaldehyde, responding to acetaldehyde, methylglyoxal, and glyoxal to far lesser degrees and not at all to a range of other aldehydes and alcohols tested. The genes frmA and frmB encode a formaldehyde dehydrogenase and S-formylglutathione hydrolase, respectively, and are responsible for detoxifying formaldehyde to formic acid in a glutathione-dependent pathway. The negative-feedback regulation of the frmRAB operon is similar to that of many other prokaryotic operons, whereby the transcription factor represses its own transcription.
Fig 1: Without Formaldehyde
Fig 2: With Formaldehyde
Features
- It’s a formaldehyde-inducible promoter from E.coli.
- It’s an engineered promoter. It retains formaldehyde responsiveness, with 2-fold higher GFP expression in response to 100 μM formaldehyde than the native pfrm. Application of this promoter with higher basal and induced expression levels before methanol assimilation genes achieves higher biomass titers than the native E. coli pfrm.
Origins
Escherichia coli
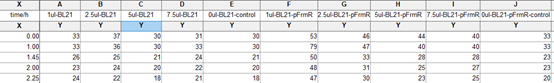
Experimental Characterization
Though we failed a lot of times that we cannot even count, due to a lot of different reasons and controls. Eventually we found the proper setting of our equipment, and got some promising data as below:
Experiment 1
Culture environment: saturated formaldehyde aqueous solution with concentration of 37 percent.
Instruments: tecan infinite m1000
Experiment group: BL21 with pFrmR+GFP
Control group: BL21 (with no plasmid transformed)
- Test the OD number of overnight-cultured strain;
- Diluted to 0.05, the strains are cultured under 37 celsius, 220prm.
- Take 5 tubes of 1ml system both from experiment group and control group and add 0ul, 1ul, 2.5ul, 5ul, and 7.5ul saturated formaldehyde solution into each tube. Mix evenly.
- ake 150ul from each of the tubes into 96 orifice plate.
- est fluorescence in tecan infinite m1000.
Related conditions:
Excitation Wavelength 495 nm
Emission Wavelength 525 nm
List of actions in this measurement script:
Shaking (Orbital) Duration: 900 s
Shaking (Orbital) Amplitude: 2 mm
Shaking (Orbital) Frequency: 306 rpm
Results:
Improvements
The sequence of this part was taken from the research of Rohlhill J. et al. The 2 binding sites of variations are -35 and -10 (Fig 1). We ordered synthesized plasmid with pFrmR and EGFP from Gensceipt and constructed pFrmR-EGFP-FrmR reporter system on plasmid pUC57. After dosing formaldehyde of 100 to 400uM, we tested the EGFP expression to identify the activity of the formaldehyde induced response of this prompter.
The research conducted by Rohlhill J. et al. has proved that, pFrmR retains formaldehyde responsiveness, with 2-fold higher GFP expression in response to 100 μM formaldehyde than the native pfrm (BBa_K749008 )[4].This is quite a new discovery and we have not found other published researches having applied this promoter, which means, we are the first team that brings it to iGEM!
Future Improvements:
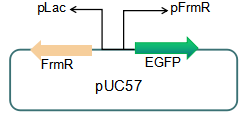
We plan to optimize our reporter vector by introducing an independent promoter pLac to regulate the expression of FrmR.
Fig 1: Sites of mutants
Fig 2: Comparison of activity
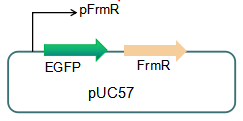
Fig 3: Current reporter system
Fig 5: Future reporter system
Potential Application
- To construct a formaldehyde sensor with this promoter.
- To enable higher growth under formaldehyde pressure with the application of the engineered formaldehyde responsive promoter.
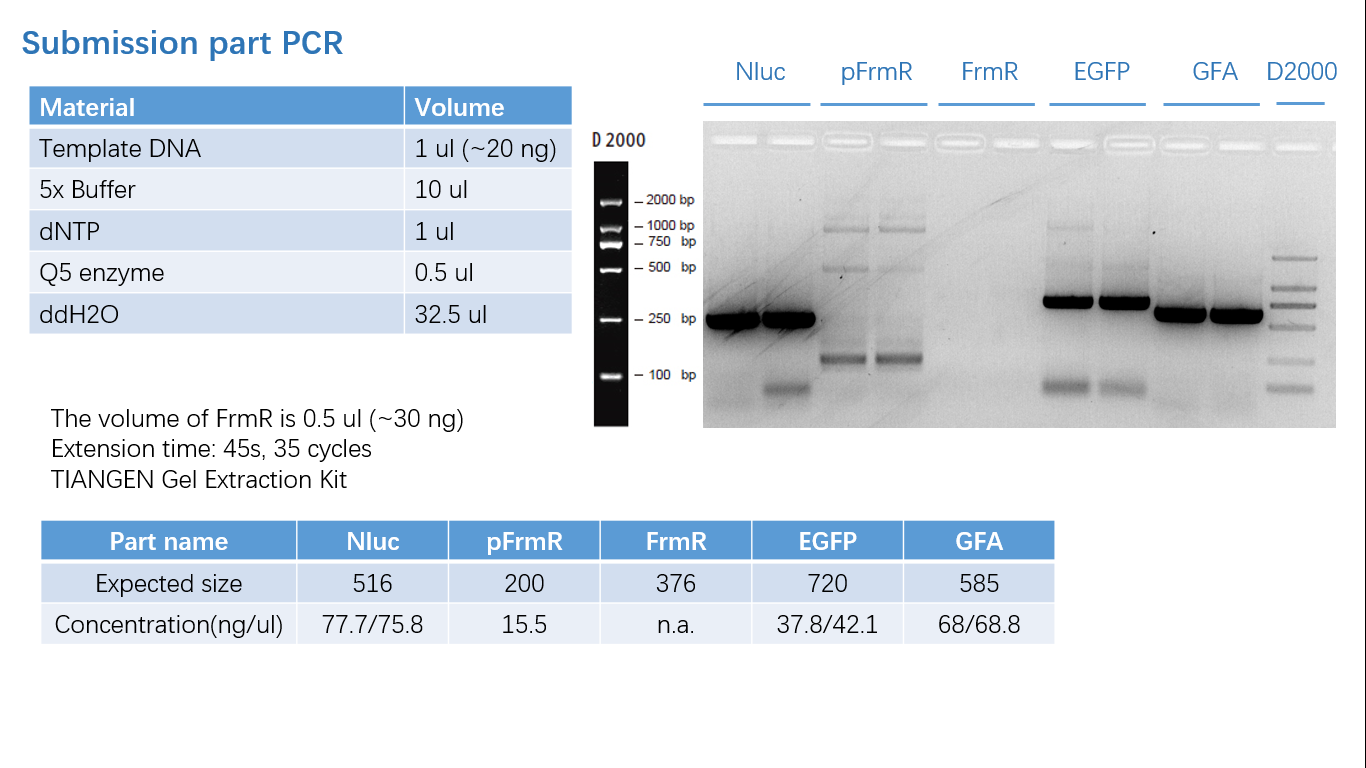
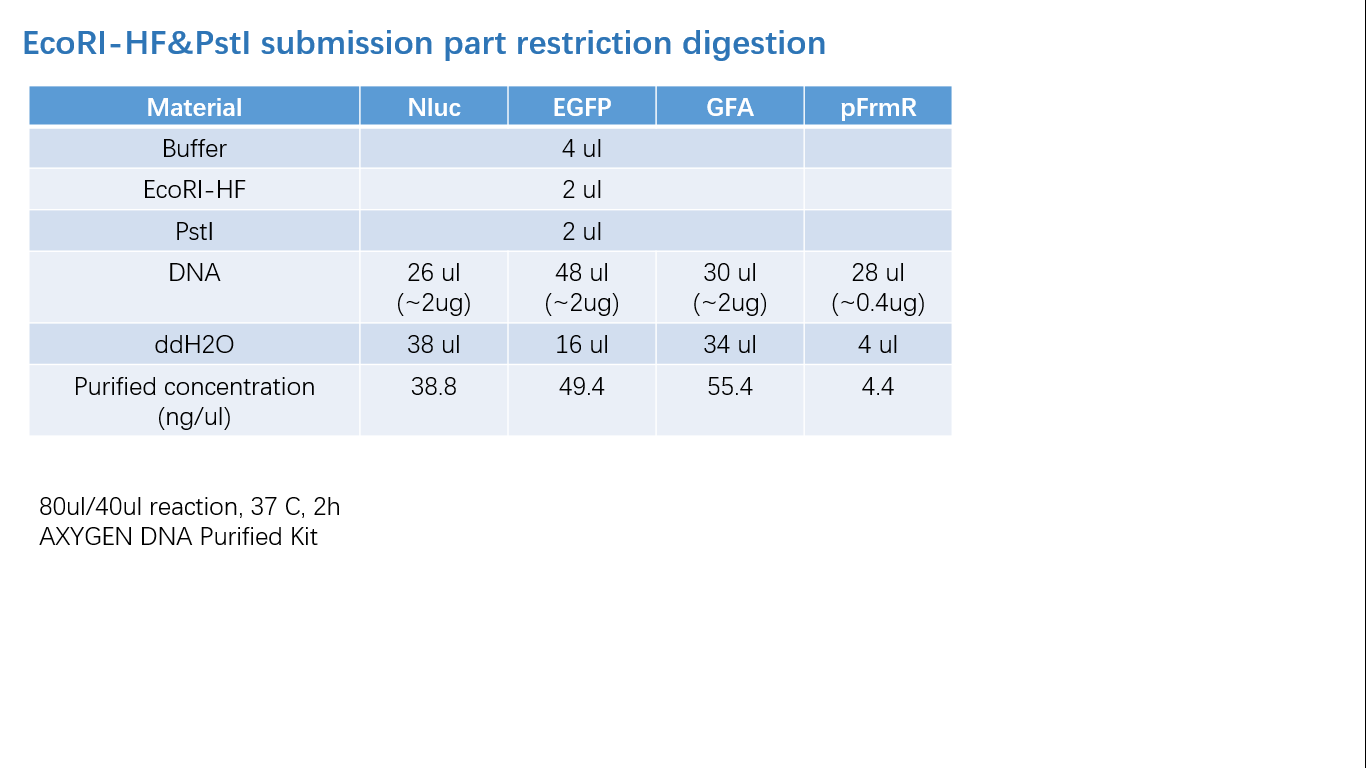
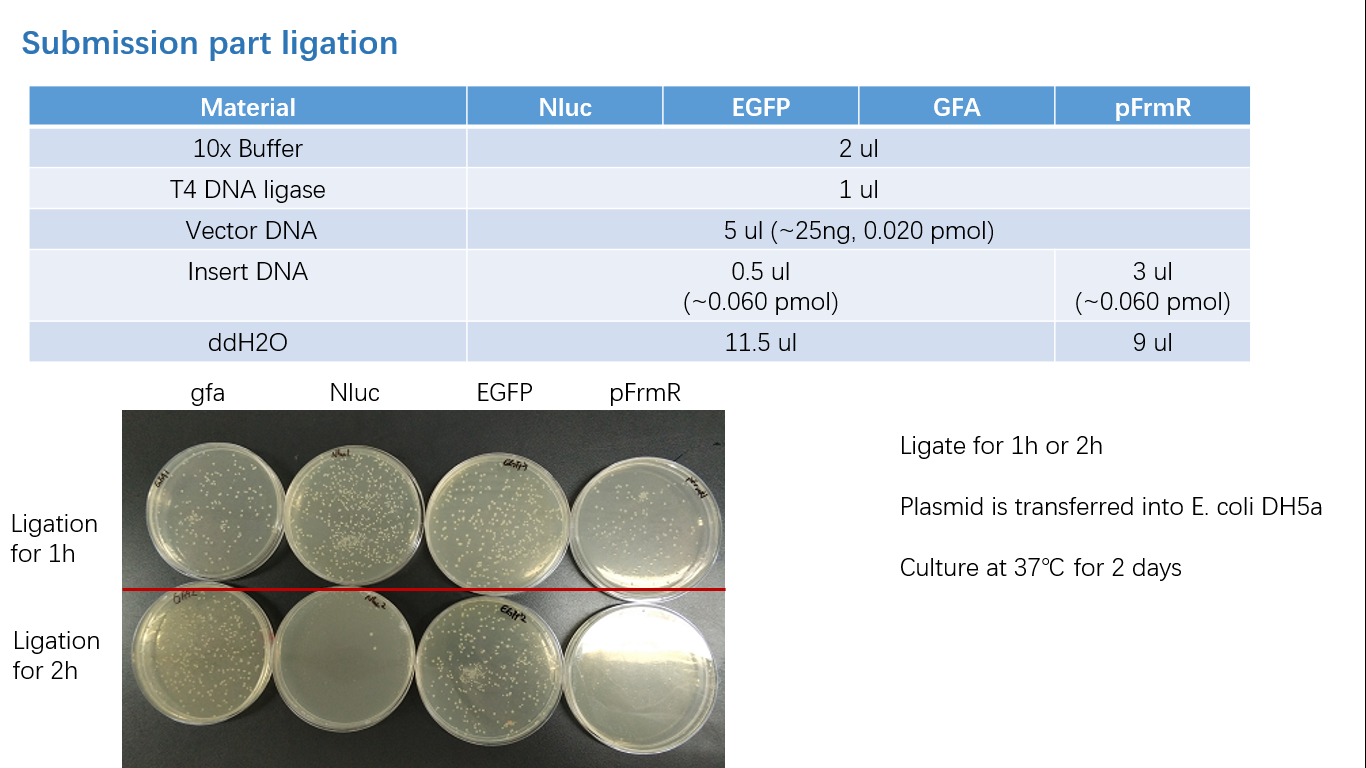
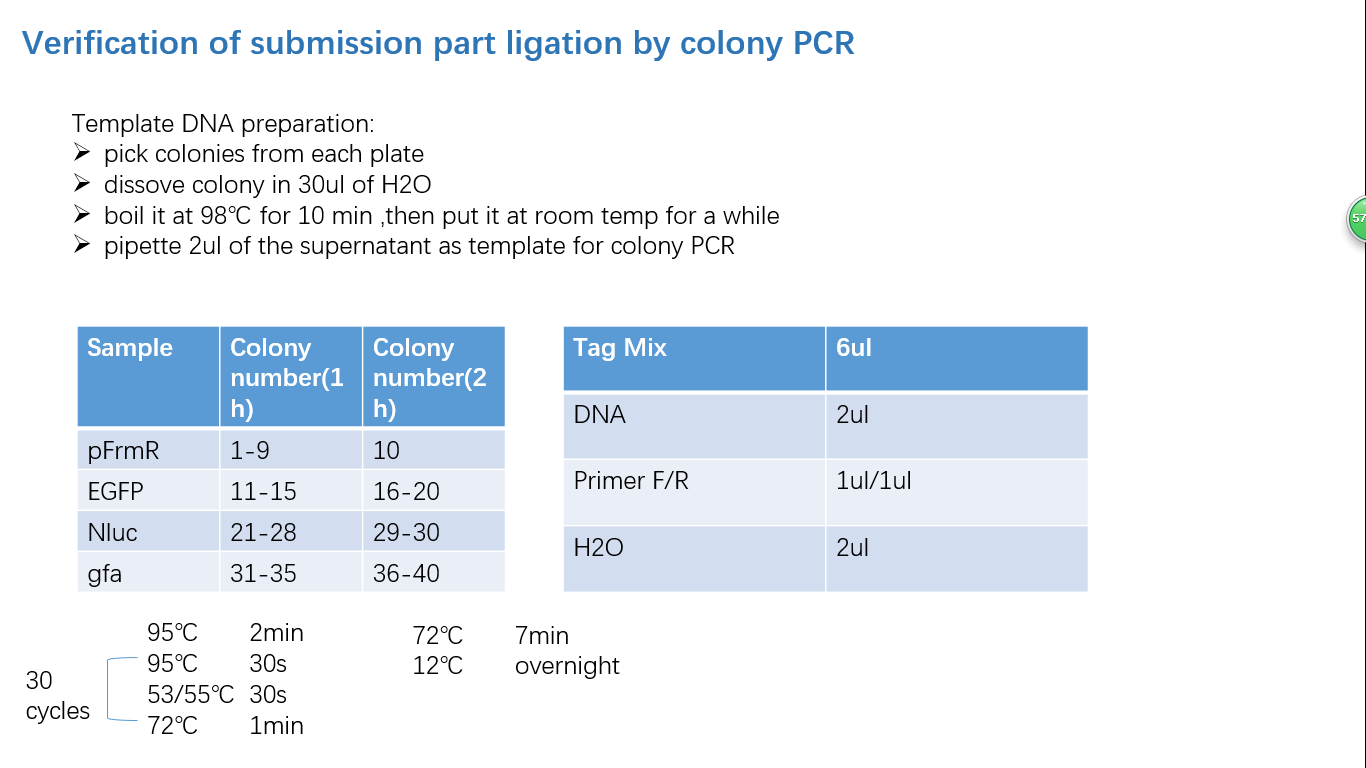
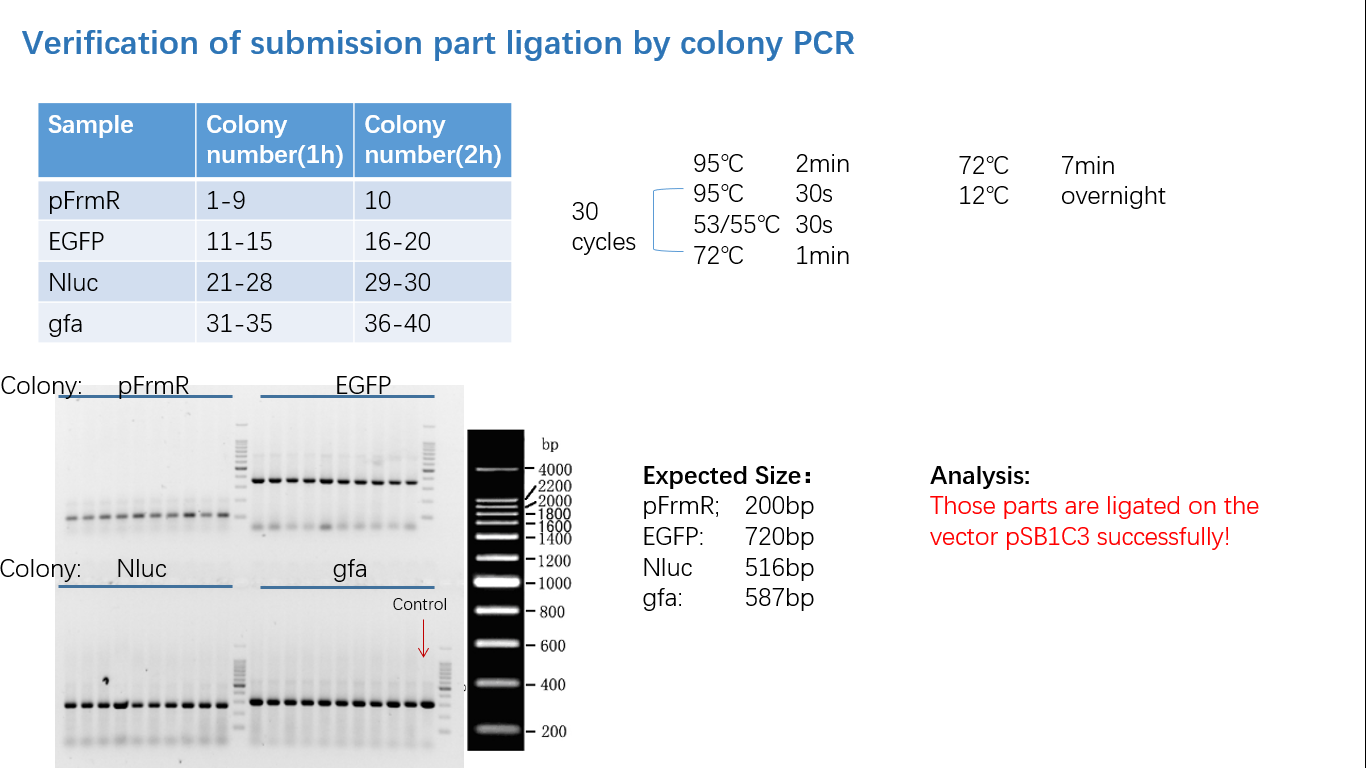
Parts Verification Before Submission
We verified our parts in the lab before submission. They are reliable! Please feel free to apply them onto your project.=)
Fig 1: PCR (to get targeted genes)
Fig 2: Restriction Digestion
Fig 3: Ligation
Fig 4: Colony PCR
Fig 5: Gel Verification
References
- Osman, D., Piergentili, C., Chen, J., Sayer, L. N., Uson, I., Huggins, T. G., Robinson, N. J., and Pohl, E. (2016) The Effectors and Sensory Sites of Formaldehyde-Responsive Regulator FrmR and Metal-Sensing Variant. J. Biol. Chem. 291, 19502-19516
- Denby, K. J., Iwig, J., Bisson, C., Westwood, J., Rolfe, M. D., Sedelnikova, S. E., Higgins, K., Maroney, M. J., Baker, P. J., Chivers, P. T., and Green, J. (2016) The mechanism of a formaldehyde-sensing transcriptional regulator. Sci. Rep. 6, 38879
- Gonzalez, C. F., Proudfoot, M., Brown, G., Korniyenko, Y., Mori, H., Savchenko, A. V., and Yakunin, A. F. (2006) Molecular basis of formaldehyde detoxification: Characterization of two S-formylglutathione hydrolases from Escherichia coli, FrmB and YeiG. J. Biol. Chem. 281, 14514-14522
- Rohlhill J, Sandoval N R, Papoutsakis E T. Sort-seq approach to engineering a formaldehyde-inducible promoter for dynamically regulated Escherichia coli growth on methanol.[J]. Acs Synthetic Biology, 2017, 6(8)
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]