Difference between revisions of "Part:BBa K2621000"
(→Example for Toehold cross-interaction determination) |
(→Example for Toehold cross-interaction determination) |
||
| Line 99: | Line 99: | ||
After the encapsulation, the droplets which have the correct Trigger RNA and Toehold Switch combination (in which Trigger RNA binds to that specific Toehold Switch), can produce catalytic biomolecules . In turn, those catalytic biomolecules can produce the <b>Product Nucleotides</b>. | After the encapsulation, the droplets which have the correct Trigger RNA and Toehold Switch combination (in which Trigger RNA binds to that specific Toehold Switch), can produce catalytic biomolecules . In turn, those catalytic biomolecules can produce the <b>Product Nucleotides</b>. | ||
| − | + | ||
| + | In other droplets, where the Trigger RNA binds to the Toehold Switch with weaker affinity, there are also less catalytic biomolecules and Product Nucleotides produced. Finally, the droplets in which the Trigger RNA does not bind to the Toehold Switch, there are no biomolecules or product nucleotides produced. | ||
=Characterization of the CAT-Seq Esterase (Vilnius-Lithuania Overgraduate 2018)= | =Characterization of the CAT-Seq Esterase (Vilnius-Lithuania Overgraduate 2018)= | ||
Revision as of 01:18, 17 October 2018
CAT-Seq Esterase
CAT-Seq Esterase is a hydrolase that was used in Catalytic Activity Sequencing system for its promiscuous capability to hydrolyse N4-acyl-2'-deoxycytidine triphosphates (Substrate Nucleotides). It has been found that the enzyme accepts various N4-acyl-2'-deoxycytidine triphosphates as substrates for hydrolysis, leaving a 2'-deoxycytidine triphosphate (Product Nucleotide).
Sequence-wise it is the most similar to tannases (EC 3.1.1.20) and feruloyl esterases (EC 3.1.1.73), however to date there has been no enzyme characterized hydrolysing N4-amidic bond in modified deoxycytidine triphosphates.
It is the main component of Catalytic Activity Sequencing (CAT-Seq) method. CAT-Seq is a method for high-throughput catalytic biomolecule and genetic regulatory part activity-sequence relationship assessment toolkit.
See how this part is used in the CAT-Seq by pressing here!
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 671
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 487
- 1000COMPATIBLE WITH RFC[1000]
Contents
Introduction
Biology
Description of the CAT-Seq esterase
CAT-Seq Esterase is a hydrolase that has a promiscuous capability to hydrolyse N4-acyl-2'-deoxycytidine triphosphates (Substrate Nucleotide). Sequence-wise it is the most similar to tannases (EC 3.1.1.20) and feruloyl esterases (EC 3.1.1.73), however to date there has been no enzyme characterized hydrolysing N4-amidic bond in modified deoxycytidine triphosphates.
To further elaborate on the fact, it has been found that the enzyme accepts various N4-acyl-2'-deoxycytidine triphosphates as substrates for hydrolysis, leaving a 2'-deoxycytidine triphosphate (Product Nucleotide).
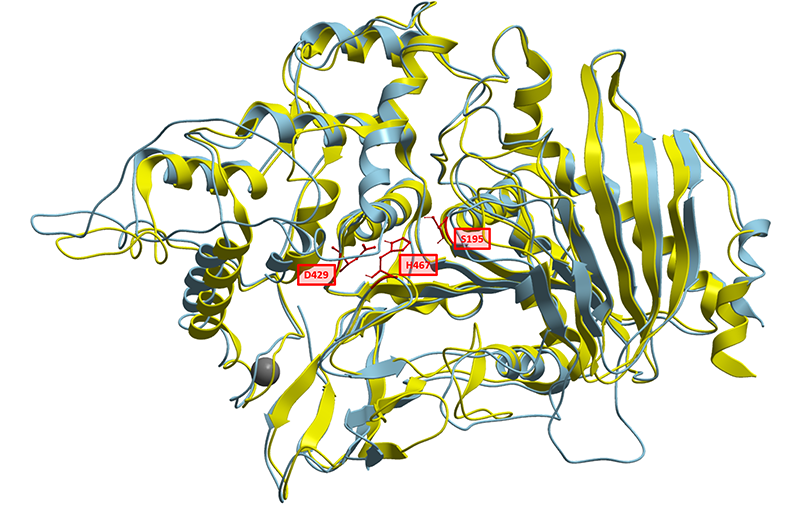
To better understand the nature of this biological part, we have generated a structural homology model based on solved structures of homologous feruloyl esterases. Currently known feruloyl esterases contain a catalytic triad in their active sites consisting of Serine, Aspartate and Histidine. Aligning CAT-Seq Esterase to the structural 3WMT template, we could also identify the catalytic triad consisting of Ser195, Asp429, His467. The sequence similarities and the positions Catalytic Triad amino acids are highlighted in the illustration below (Fig. 1).
In-Silico design of the CAT-Seq esterase mutants
The reason of making esterase mutants was to benchmark the accuracy of CAT-Seq system. We have aimed to make Esterase mutants that would have slightly different activities. After deriving the mutations, we have measured their activities using standard methods, and then compared them with CAT-Seq measurements. Therefore, the esterase and its mutants can now be used for accuracy calibration when setting up the CAT-Seq for the first time.
The residues chosen for mutation were scattered around the catalytic triad of the esterase and involved mainly polar and aromatic side chains. In literature, some of these side chains are assumed to form bonds stabilizing the binding of the substrate (Fig. 2).

After carefully selecting the mutations in-silico, we have synthesized and measured the Activity of those esterase mutants in the laboratory. The mutations have successfully altered the activity of the original Esterase. Also, there were no cases of complete inactivation of enzyme catalytic activity from the mutants we managed test. Please head to the results section for further information.
Usage with CAT-Seq (Catalytic Activity Sequencing)
About CAT-Seq
CAT-Seq stands for Catalytic Activity Sequencing - a system designed and built for high-speed, simultaneous characterization of Catalytic and Regulatory biological parts. You can learn more about CAT-Seq [http://2018.igem.org/Team:Vilnius-Lithuania-OG by clicking this link]
Determining the accuracy of CAT-Seq
Trying to build a CAT-Seq pipeline in your own laboratory will require the CAT-Seq esterase in order to troubleshoot the system and assess the measurement accuracy and precision. In other words, the esterase can be used to calibrate the CAT-Seq.
Together with the Esterase, its substrate attached to a nucleotide is required (substrate nucleotide). In the standard case, the Substrate Nucleotide is N4-benzoyl-2'-deoxycytidine triphosphate. If the Esterase catalyzes the removal of the substrate from the nucleotide, it becomes the Product Nucleotide - 2'-deoxycytidine triphosphate.
Genetic Regulatory Part activity and cross-interaction assessment
While Catalytic Activity Sequencing began as a method for catalytic biomolecule activity recording, we have also create a way to adjust CAT-Seq to record activities of regulatory part. In addition to the activities, cross-interactions of different regulatory parts can also be measured.
When assessing the activities and sequences of libraries of catalytic biomolecules in CAT-Seq , the activity is measured and recorded as a function of Product Nucleotide that was produced in each droplet.
Yet, the activity of the catalytic biomolecule is not the only aspect that can influence the amount of Product Nucleotides that are produced. If all of the droplets would contain the same catalytic biomolecule , but each droplet would have a different concentration of that biomolecule, we would in result get different amounts of Product Nucleotides. For example, droplets with large amount of biomolecules may produce a large number of Product Nucleotides and vice-versa. The default and well-characterized Catalytic Biomolecule in CAT-Seq for regulatory part charectation would be the CAT-Seq Esterase.
Yet, the activity of the catalytic biomolecule is not the only aspect that can influence the amount of Product Nucleotides that are produced. If all of the droplets would contain the same catalytic biomolecule , but each droplet would have a different concentration of that biomolecule, we would in result get different amounts of Product Nucleotides. For example, droplets with large amount of biomolecules may produce a large number of Product Nucleotides and vice-versa. The default and well-characterized Catalytic Biomolecule in CAT-Seq for regulatory part charectation would be the CAT-Seq Esterase.
Example for RBS activity strength determination
We can now explore an example in which the CAT-Seq esterase can be used to assess the strengths of different ribosome binding site (RBS) sequences.
While keeping all the workflow from the main Catalytic Activity Sequencing design, all we need to do is to change the way we prepare the library. Firstly, instead of using a library of catalytic biomolecules, we can use a single CAT-Seq Esterase enzyme. Then, specific or random RBS sequence library can be prepared and attached to the esterase enzyme sequence. After that, every step of CAT-Seq method is the same.
After the library is encapsulated into droplets, depending on the RBS sequence strength some droplets may have different amounts of the esterase. For example, the droplets with more esterase will produce more Product Nucleotides and vice-versa. After the amplification and sequencing steps we can learn two main things about the measured library - the RBS sequence and its relative RBS strength in the form of Product Nucleotide concentration for every library variant screened.
Example for Toehold cross-interaction determination
Next, we want to give an example on how to record regulatory part interactions using CAT-Seq. In this case, we will be using Toehold Switches.
The Toehold Switch systems are composed of two RNA strands referred to as the Switch and Trigger. The Switch RNA contains the coding sequence of the gene being regulated. The Switch RNA forms a hairpin structure that includes the RBS site. While the hairpin structure is formed, the translation is inhibited. The Trigger RNA is a molecule that can selectively bind to the Switch RNA region and expose the gene RBS site for ribosomes. Once that happens, the protein translation can be initiated.
Once again, only the first part of general CAT-Seq design needs to be changed - the library preparation. Instead of using a catalytic biomolecule library, a single enzyme is used. Then, libraries need to be prepared - one for Toehold Switches and another for Trigger RNA. The Trigger RNA libraries also require a separate T7 promoter for RNA expression. Then, both of those libraries must be ligated to the enzyme DNA fragment. Resulting library contains fragments which have the same catalytic biomolecule (In the general case - the CAT-Seq Esterase), yet each of them have a random combination of a specific Toehold Switch and RNA Trigger.
After the encapsulation, the droplets which have the correct Trigger RNA and Toehold Switch combination (in which Trigger RNA binds to that specific Toehold Switch), can produce catalytic biomolecules . In turn, those catalytic biomolecules can produce the Product Nucleotides.
In other droplets, where the Trigger RNA binds to the Toehold Switch with weaker affinity, there are also less catalytic biomolecules and Product Nucleotides produced. Finally, the droplets in which the Trigger RNA does not bind to the Toehold Switch, there are no biomolecules or product nucleotides produced.
Characterization of the CAT-Seq Esterase (Vilnius-Lithuania Overgraduate 2018)
Esterase and its mutant activity measurement
Challenges of inactivating RNA I in wild type origin of replication were discussed earlier
After the construction of selected ColE1 mutants with inactivated RNA I promoter we have tested whether it was successful. It is difficult to distinguish when the promoter is fully disabled because first, there is no literature data describing replicons that are not negatively regulated at least to some extent, and second - plasmid systems hardly reach the equilibrium without negative control therefore every copy number calculation varies greatly. This is why we decided not to check for the highest copy number mutant, but rather to insert a wild type RNA I with its wild type promoter. By doing that we could see which replicons were most precisely mutated.
ORI 2 mutant seemed like a perfect candidate. Its copy number increased from wild type 37 copies to 1128 ± 315 copies in ORI2. In addition, when RNA I gene was placed next to it, the copy number of the constructed plasmid fell to wild type levels. After these results we have decided to use this ORI 2 mutant as a core for our framework. We simply called it RNA II (part:BBa_K2259000)
In silico produced mutant library based on CAT-Seq esterase enzyme
Once the RNA I promoter was disabled in the ColE1 origin of replication, it could be moved to a different plasmid location and used as a separate unit. We have discovered the sequence of wild type RNA I promoter by using PromoterHunter and removed it, thus creating a wild type RNA I gene part:BBa_K2259005. First, series of Anderson promoters were cloned next to the RNA I gene (part:BBa_K2259021 (0.15 Anderson), part:BBa_K2259023 (0.36 Anderson), part:BBa_K2259027 (0.86 Anderson), part:BBa_K2259028 (1.0 Anderson)) and then placed next to RNA II (part:BBa_K2259067 (0.15 Anderson), part:BBa_K2259068 (0.36 Anderson), part:BBa_K2259069 (0.86 Anderson), part:BBa_K22590671 (1.0 Anderson)).
Ribosome binding sites
When different groups of SynORI system were created, the abilty of corresponding RNA I to inhibit the replication of RNA II were measured by calculating the plasmid copy number with and without RNA I in the system
Toehold regulatory sequence library
As can be seen in Figure 7, RNA I introduction into the system has a significant effect on the plasmid copy number of the specific group, thus we can conclude that RNA I works on corresponding RNA II.