Difference between revisions of "Part:BBa K2596003"
(→Luciferase Assays) |
(→Luciferase Assays) |
||
| Line 28: | Line 28: | ||
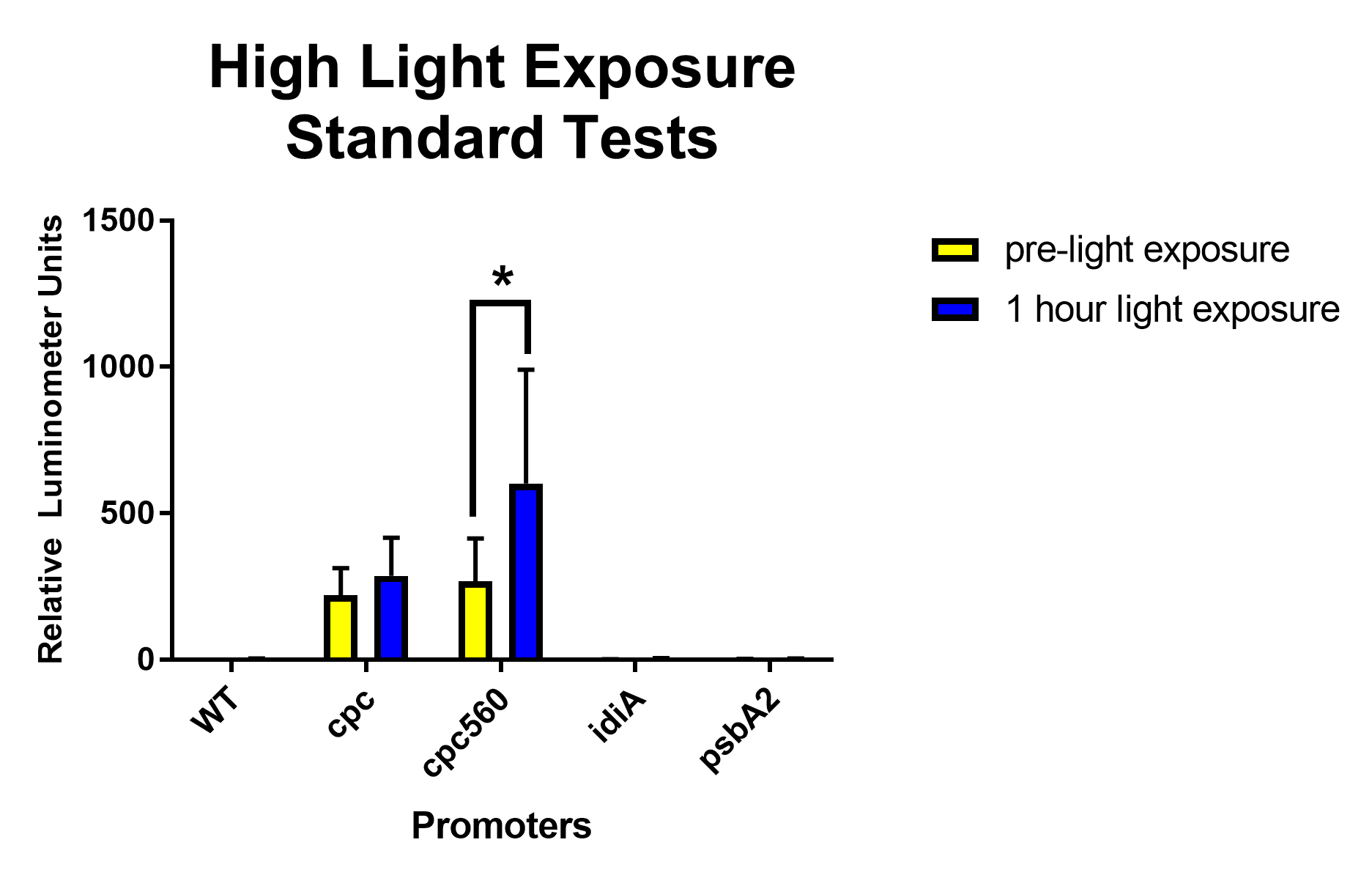
[[File: T--Stony Brook--Results high light exposure.png|thumb|Figure 3. High light exposure Standard Expression tests for the constitutive promoters cpc and cpc-560, ferrous ion repressible idiA, and high light inducible psbA2 promoters]] | [[File: T--Stony Brook--Results high light exposure.png|thumb|Figure 3. High light exposure Standard Expression tests for the constitutive promoters cpc and cpc-560, ferrous ion repressible idiA, and high light inducible psbA2 promoters]] | ||
| + | |||
| + | Experimental Methods: | ||
In order to test the expression of our promoters, cpc (BBa_K2596001), cpc560 (BBa_K2596006), idiA (BBa_K2596004), psbA2 (BBa_K2596003), which were incorporated into Dr. Susan Golden’s vector pAM1414 using Gibson assembly, we conducted luciferase experiments. Following Dr. Golden’s procedure, we added 5 µL of decanal to 95 µL of cyanobacteria in each well to induce expression. The decanal acted as a substrate for the bacterial luciferase enzyme, but due to the toxicity, the cells ended up dying, so the data obtained represents the end static expression. For the standard expression experiments for nighttime and daytime, we plated 95 µL of cyanobacteria into a 96 well plate, added 5 µL of decanal, parafilmed the edges and left the plate for 15 minutes before measuring the luminescence in a plate reader. For the psbA2 experiment, we plated 95 µL of cyanobacteria to half of the wells and put them under at least 500 µE of high light in our incubator for 1 hour. Then, we added 95 µL of cyanobacteria not exposed to high light to the other half of the wells and added 5 µL of decanal to all of the wells. Then, we placed the well plate in a plate reader and measured the luminescence. | In order to test the expression of our promoters, cpc (BBa_K2596001), cpc560 (BBa_K2596006), idiA (BBa_K2596004), psbA2 (BBa_K2596003), which were incorporated into Dr. Susan Golden’s vector pAM1414 using Gibson assembly, we conducted luciferase experiments. Following Dr. Golden’s procedure, we added 5 µL of decanal to 95 µL of cyanobacteria in each well to induce expression. The decanal acted as a substrate for the bacterial luciferase enzyme, but due to the toxicity, the cells ended up dying, so the data obtained represents the end static expression. For the standard expression experiments for nighttime and daytime, we plated 95 µL of cyanobacteria into a 96 well plate, added 5 µL of decanal, parafilmed the edges and left the plate for 15 minutes before measuring the luminescence in a plate reader. For the psbA2 experiment, we plated 95 µL of cyanobacteria to half of the wells and put them under at least 500 µE of high light in our incubator for 1 hour. Then, we added 95 µL of cyanobacteria not exposed to high light to the other half of the wells and added 5 µL of decanal to all of the wells. Then, we placed the well plate in a plate reader and measured the luminescence. | ||
| + | |||
| + | Results: | ||
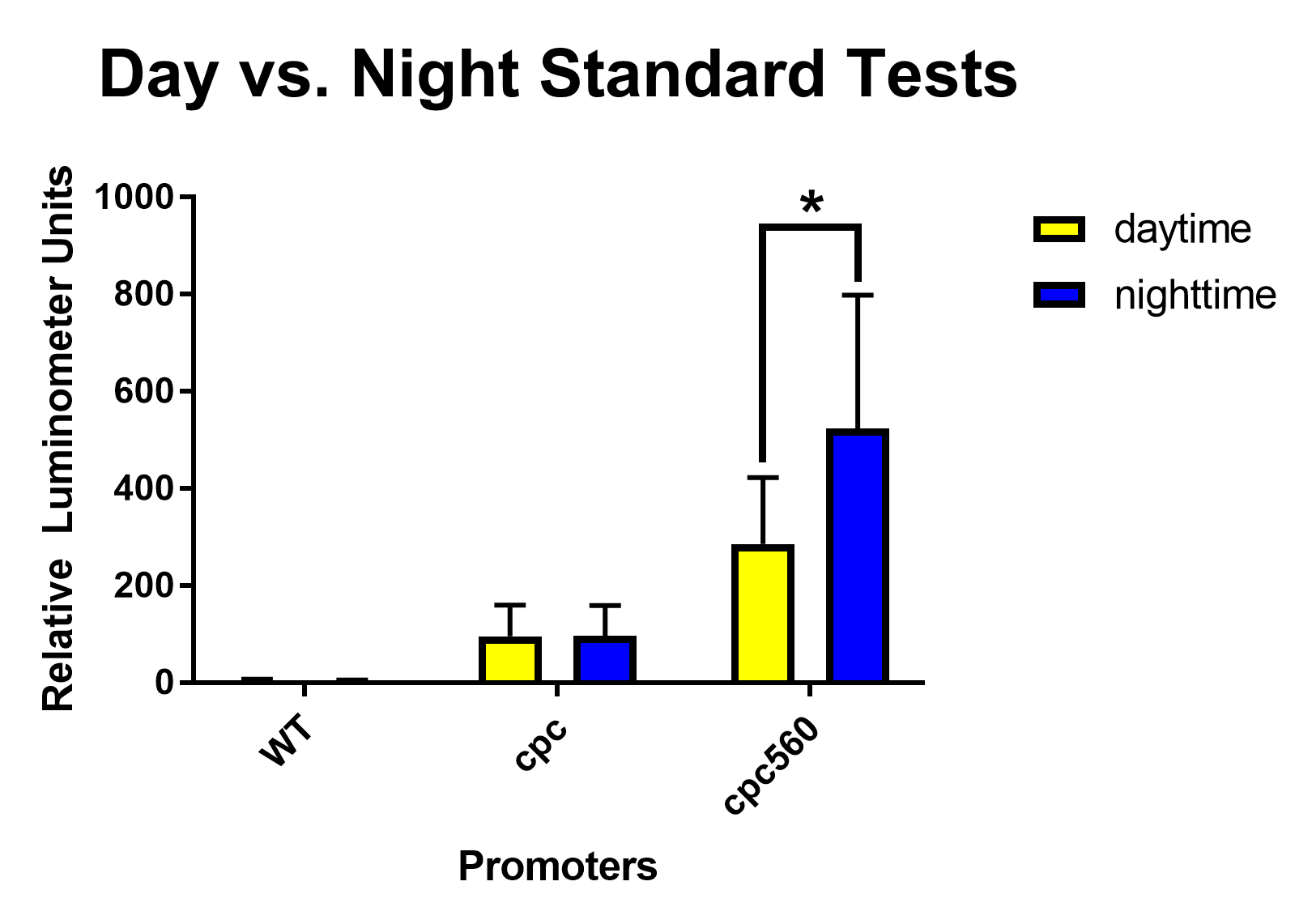
After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, both cpc and cpc-560 showed significant differences for daytime expression compared to wild type [Figure 1]. Compared to cpc, cpc-560 had a significantly higher expression [Figure 1]. In comparison for day compared to night, cpc did not show any significant difference [Figure 2]. On the other hand, cpc-560 showed a significant difference between daytime and nighttime expression [Figure 2]. | After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, both cpc and cpc-560 showed significant differences for daytime expression compared to wild type [Figure 1]. Compared to cpc, cpc-560 had a significantly higher expression [Figure 1]. In comparison for day compared to night, cpc did not show any significant difference [Figure 2]. On the other hand, cpc-560 showed a significant difference between daytime and nighttime expression [Figure 2]. | ||
After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, for daytime expression, compared to all other promoters and the wild type, cpc and cpc-560 had significant differences in expression [Figure 1]. For idiA and psbA2, they only had significant differences compared to cpc and cpc-560, and not to each other [Figure 1]. When exposed to highlight, idiA and psbA2 did not show any expression [Figure 3]. | After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, for daytime expression, compared to all other promoters and the wild type, cpc and cpc-560 had significant differences in expression [Figure 1]. For idiA and psbA2, they only had significant differences compared to cpc and cpc-560, and not to each other [Figure 1]. When exposed to highlight, idiA and psbA2 did not show any expression [Figure 3]. | ||
Revision as of 16:53, 16 October 2018
PpsbA2 from Synechococcus elongatus PCC 7942
This a high-light inducible promoter from cyanobacteria Synechococcus elongatus PCC 7942
Usage and Biology
PpsbA2 is a light-inducible promoter native to cyanobacteria. Specifically, it comes from a strain (Synechococcus elongatus PCC 7942) closely related to our chassis organism. The promoter is activated by high light intensity or blue light, and deactivated by low light intensity or red light.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
The promoter contains a conserved AT-box motif just upstream of the ribosome binding site that serves as the binding site for light-activated proteins. For the Biobrick, we kept most of the original promoter, including a crucial upstream element and the core promoter sequence. Our final Biobrick is 157 bp in length.
Luciferase Assays
Experimental Methods:
In order to test the expression of our promoters, cpc (BBa_K2596001), cpc560 (BBa_K2596006), idiA (BBa_K2596004), psbA2 (BBa_K2596003), which were incorporated into Dr. Susan Golden’s vector pAM1414 using Gibson assembly, we conducted luciferase experiments. Following Dr. Golden’s procedure, we added 5 µL of decanal to 95 µL of cyanobacteria in each well to induce expression. The decanal acted as a substrate for the bacterial luciferase enzyme, but due to the toxicity, the cells ended up dying, so the data obtained represents the end static expression. For the standard expression experiments for nighttime and daytime, we plated 95 µL of cyanobacteria into a 96 well plate, added 5 µL of decanal, parafilmed the edges and left the plate for 15 minutes before measuring the luminescence in a plate reader. For the psbA2 experiment, we plated 95 µL of cyanobacteria to half of the wells and put them under at least 500 µE of high light in our incubator for 1 hour. Then, we added 95 µL of cyanobacteria not exposed to high light to the other half of the wells and added 5 µL of decanal to all of the wells. Then, we placed the well plate in a plate reader and measured the luminescence.
Results:
After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, both cpc and cpc-560 showed significant differences for daytime expression compared to wild type [Figure 1]. Compared to cpc, cpc-560 had a significantly higher expression [Figure 1]. In comparison for day compared to night, cpc did not show any significant difference [Figure 2]. On the other hand, cpc-560 showed a significant difference between daytime and nighttime expression [Figure 2].
After removing outliers from the data set using the 1.5(IQR) rule and conducting unpaired T-tests assuming unequal variance, for daytime expression, compared to all other promoters and the wild type, cpc and cpc-560 had significant differences in expression [Figure 1]. For idiA and psbA2, they only had significant differences compared to cpc and cpc-560, and not to each other [Figure 1]. When exposed to highlight, idiA and psbA2 did not show any expression [Figure 3].