Difference between revisions of "Part:BBa K2728004"
Bluepumpkin (Talk | contribs) |
Bluepumpkin (Talk | contribs) (→Experimental Characterization) |
||
| Line 29: | Line 29: | ||
<br /> | <br /> | ||
=== Experimental Characterization === | === Experimental Characterization === | ||
| − | + | Culture environment: saturated formaldehyde aqueous solution with concentration of 37 percent<br /> | |
| + | Instruments: tecan infinite m1000<br /> | ||
| + | Experiment group: BL21 with pFrmR+GFP and BL21 with GFA in ratio of 1:1<br /> | ||
| + | Control group A: BL21<br /> | ||
| + | Control group B: BL21 with pFrmR+EGFP<br /> | ||
<br /> | <br /> | ||
| + | #Test the OD number of overnight-cultured strain; | ||
| + | #Diluted to 0.05, the strains are cultured under 37 celsius, 220prm. | ||
| + | For control groups:<br /> | ||
| + | #Take 5 tubes of 1ml system both from the control groups and add 0.5ul saturated formaldehyde solution into each tube. Mix evenly. | ||
| + | For experiment group:<br /> | ||
| + | #Take 500ul from each strain and mix them together. | ||
| + | #Add 0.5ul of saturated formaldehyde aqueous solution. | ||
<br /> | <br /> | ||
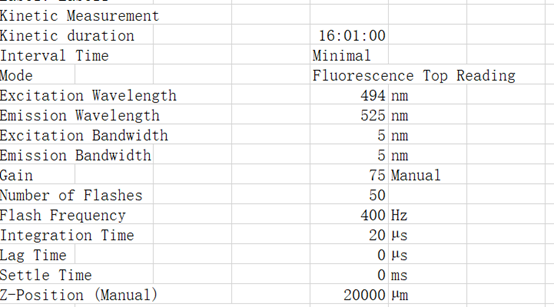
| + | Related conditions:<br /> | ||
| + | Excitation Wavelength 495 nm<br /> | ||
| + | Emission Wavelength 525 nm<br /> | ||
| + | List of actions in this measurement script:<br /> | ||
| + | Shaking (Orbital) Duration: 900 s<br /> | ||
| + | Shaking (Orbital) Amplitude: 2 mm<br /> | ||
| + | Shaking (Orbital) Frequency: 306 rpm<br /> | ||
| + | [[File:Pfmxrxp3.png|left|border|800px]]<br clear=all> | ||
| + | <br /> | ||
| + | Results:<br /> | ||
| + | [[File:T--BGIC-Global--GFA.png|left|border|500px]]<br clear=all> | ||
| + | The figure indicates that the experiment expressed the highest amount of fluorescence.<br /> | ||
| + | The experiment only had half amount of EGFP bacteria compared with the control group B, but produced higher expression. The only way to explain this result is that GFA played an important role to degrade the formaldehyde, therefore decreased the toxicity of the environment of bacteria and enhance the survival rate. This also indicates that our engineered system shown below works. Due to the time limitation, we only successfully finished only one trial. We will test more combination of mixed ratio and formaldehyde concentrations in the coming days. | ||
| + | <br /> | ||
| + | [[File:T--BGIC-Global--circuitmix.png|left|border|800px]]<br clear=all> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | |||
=== Parts Verification Before Submission === | === Parts Verification Before Submission === | ||
We verified our parts in the lab before submission. They are reliable! Please feel free to apply them onto your project.=) | We verified our parts in the lab before submission. They are reliable! Please feel free to apply them onto your project.=) | ||
Revision as of 17:48, 17 October 2018
GFA - A Glutathione-Dependent Formaldehyde-Activating Enzyme
Basic Description
An enzyme from Paracoccus denitrificans that accelerates this spontaneous condensation reaction, which catalyze the conversion of formaldehyde and glutathione was purified and named glutathione-dependent formaldehyde-activating enzyme (Gfa).
The gene GFA is located directly upstream of the gene for glutathione-dependent formaldehyde dehydrogenase, which catalyzes the subsequent oxidation of S-hydroxymethylglutathione. The glutathione-dependent formaldehyde conversion to formate starts with the adduct formation, formaldehyde reacts with the SH group of glutathione producing S-hydroxymethylglutathione (Reaction 1).
Fig 1: Thiol-dependent pathway
Formaldehyde-converting enzymes-Gfa is composed of one type of subunit of about 20 kDa and lack a chromophoric prosthetic group. In addition, both enzymes are encoded next to genes for enzymes involved in further oxidation of the cofactor-bound one-carbon unit to carbon dioxide.
Fig 2: 3D structure (from www.uniprot.org)
Sequence
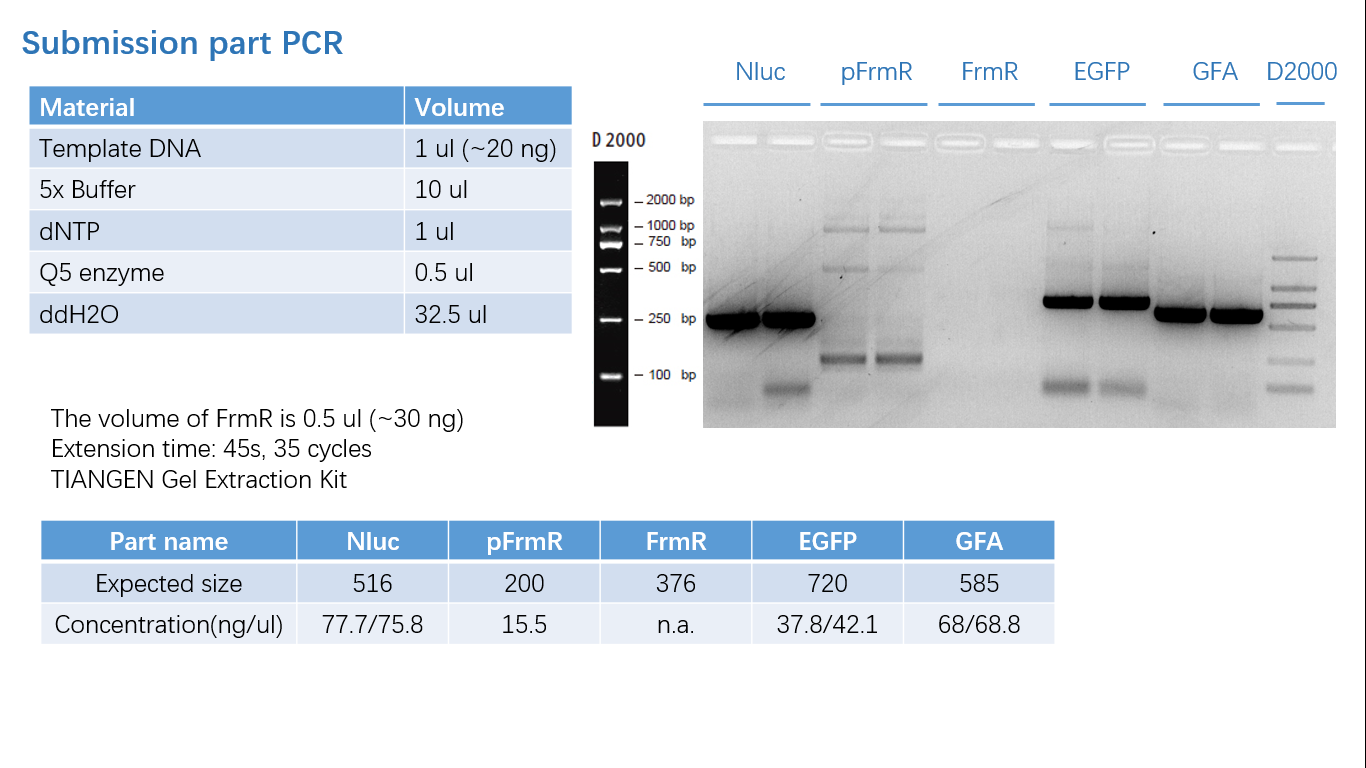
We ordered the synthesized plasmid from Genscript. After restriction digestion & transformation, we got our E. coli with GFA.
Fig 3: Order info
Origin
This gene originated from P. denitrificans. Putative proteins with sequence identity to GFA from P. denitrificans are present also in Rhodobacter sphaeroides, Sinorhizobium meliloti, and Mesorhizobium loti.
Experimental Characterization
Culture environment: saturated formaldehyde aqueous solution with concentration of 37 percent
Instruments: tecan infinite m1000
Experiment group: BL21 with pFrmR+GFP and BL21 with GFA in ratio of 1:1
Control group A: BL21
Control group B: BL21 with pFrmR+EGFP
- Test the OD number of overnight-cultured strain;
- Diluted to 0.05, the strains are cultured under 37 celsius, 220prm.
For control groups:
- Take 5 tubes of 1ml system both from the control groups and add 0.5ul saturated formaldehyde solution into each tube. Mix evenly.
For experiment group:
- Take 500ul from each strain and mix them together.
- Add 0.5ul of saturated formaldehyde aqueous solution.
Related conditions:
Excitation Wavelength 495 nm
Emission Wavelength 525 nm
List of actions in this measurement script:
Shaking (Orbital) Duration: 900 s
Shaking (Orbital) Amplitude: 2 mm
Shaking (Orbital) Frequency: 306 rpm
Results:
The figure indicates that the experiment expressed the highest amount of fluorescence.
The experiment only had half amount of EGFP bacteria compared with the control group B, but produced higher expression. The only way to explain this result is that GFA played an important role to degrade the formaldehyde, therefore decreased the toxicity of the environment of bacteria and enhance the survival rate. This also indicates that our engineered system shown below works. Due to the time limitation, we only successfully finished only one trial. We will test more combination of mixed ratio and formaldehyde concentrations in the coming days.
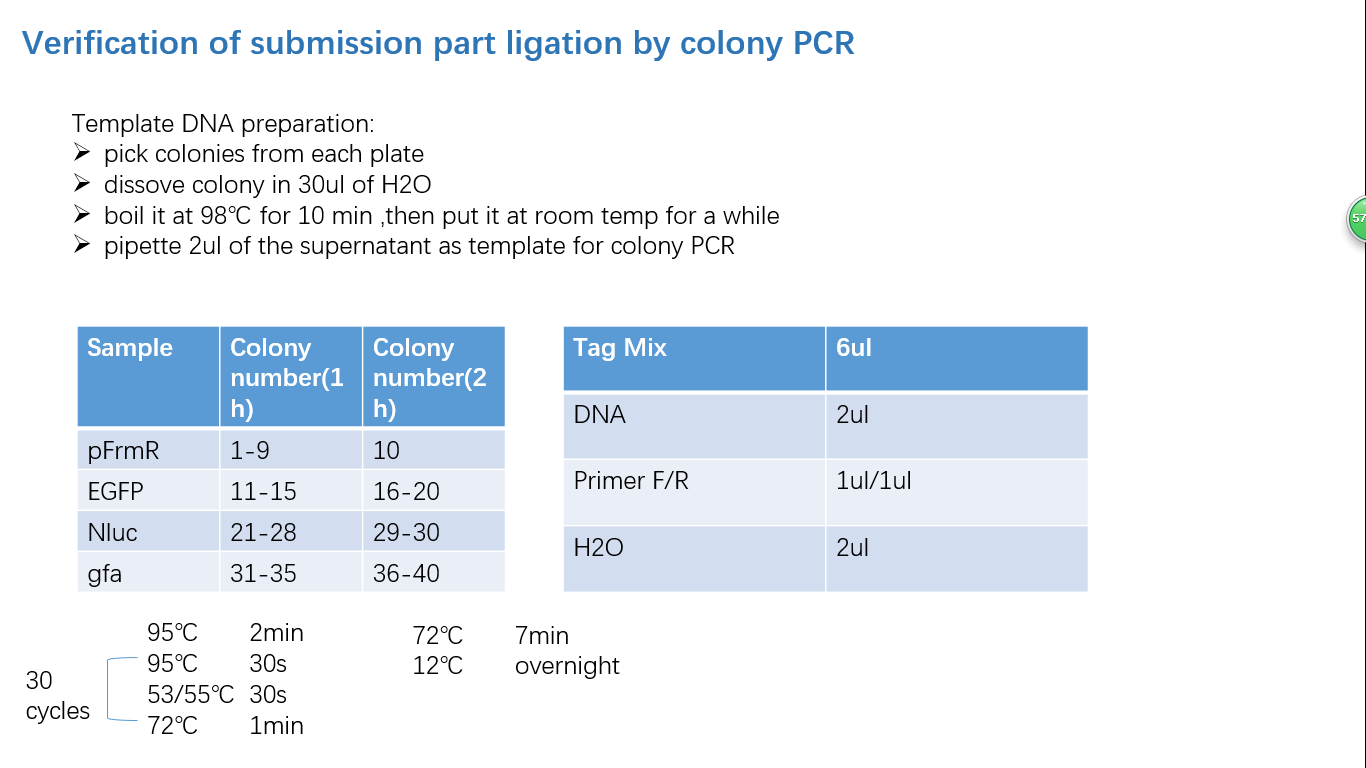
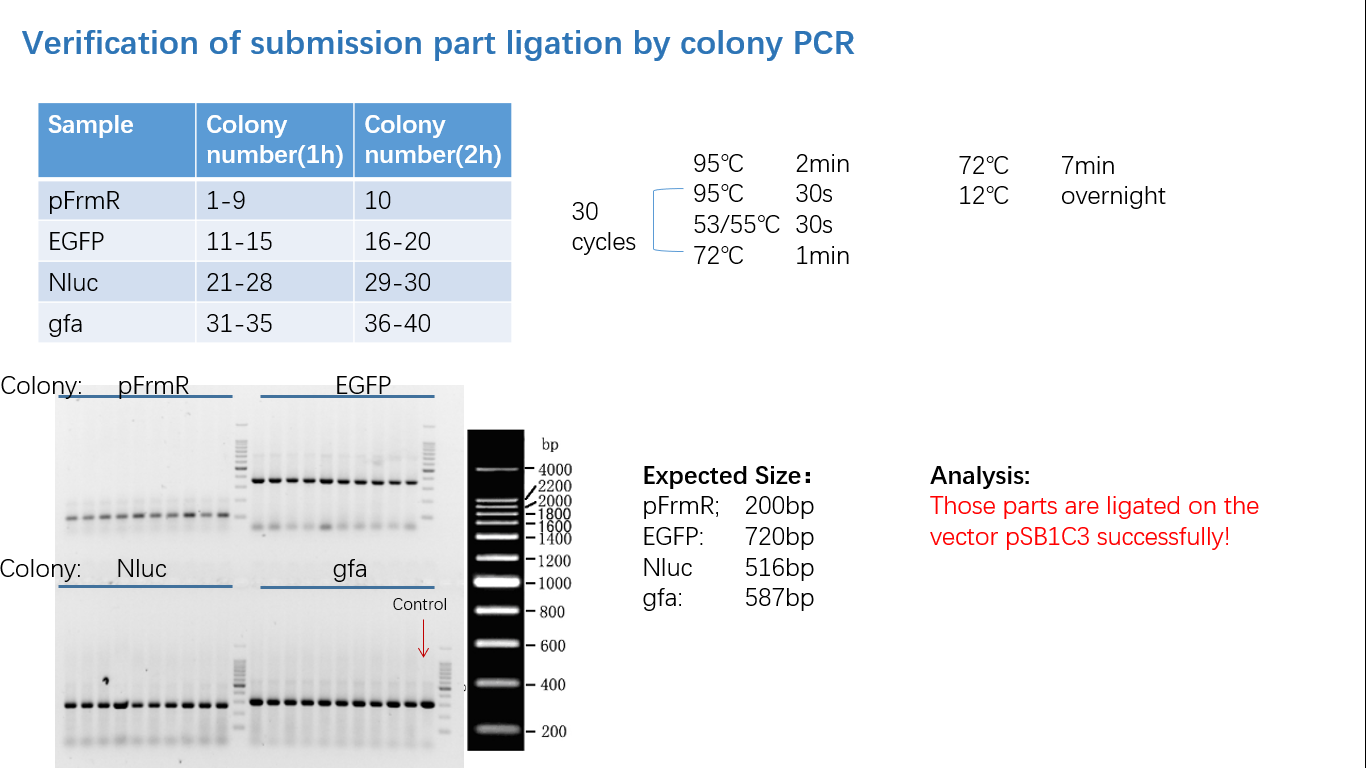
Parts Verification Before Submission
We verified our parts in the lab before submission. They are reliable! Please feel free to apply them onto your project.=)
Fig 1: PCR (to get targeted genes)
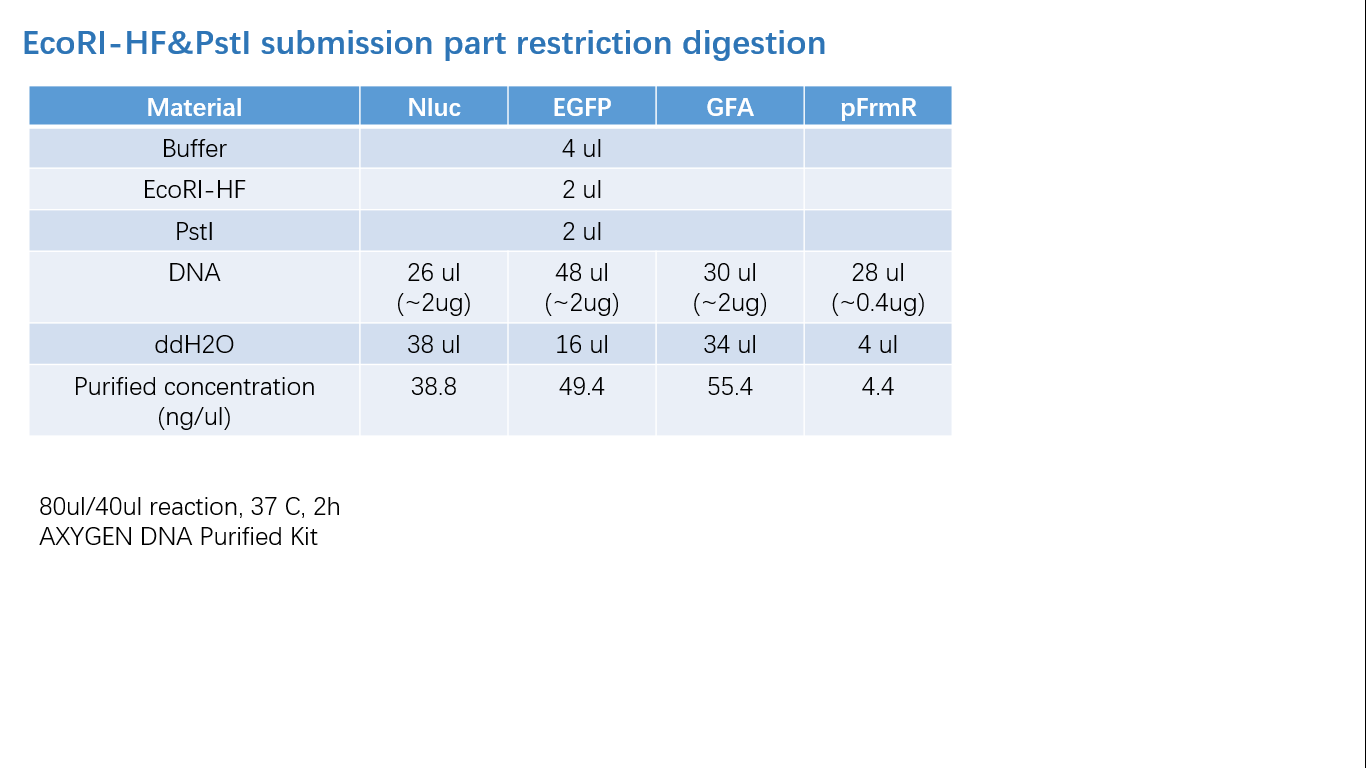
Fig 2: Restriction Digestion
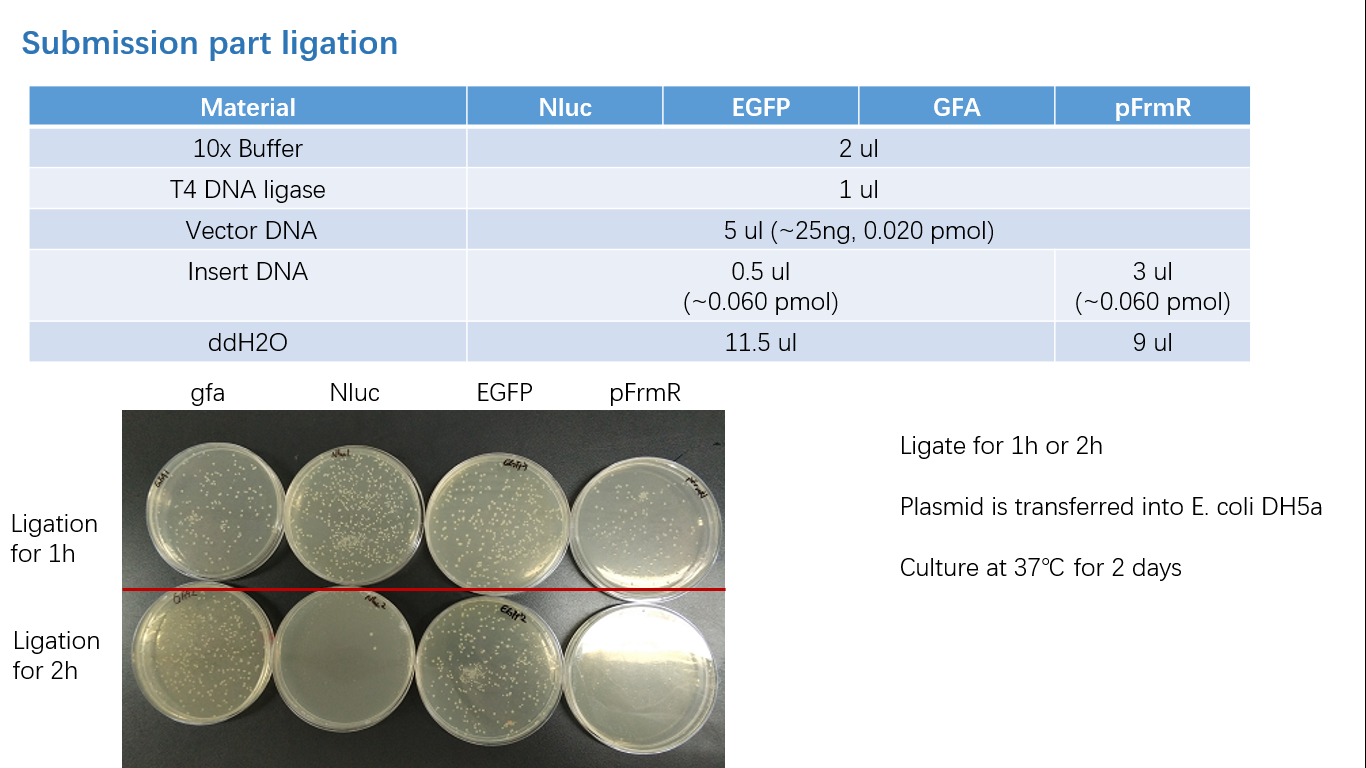
Fig 3: Ligation
Fig 4: Colony PCR
Fig 5: Gel Verification
References
- Gonzalez, C. F., Proudfoot, M., Brown, G., Korniyenko, Y., Mori, H., Savchenko, A. V., & Yakunin, A. F. (2006). Molecular Basis of Formaldehyde Detoxification. Journal of Biological Chemistry, 281(20), 14514–14522.
- Goenrich, M., Bartoschek, S., Hagemeier, C. H., Griesinger, C. & Vorholt, J. A. (2002). A glutathione-dependent formaldehydeactivating enzyme (Gfa) from Paracoccus denitrificans detected and purified via two-dimensional proton exchange NMR spectroscopy.J Biol Chem 277, 3069–3072.
- Rohlhill J, Sandoval N R, Papoutsakis E T. Sort-seq approach to engineering a formaldehyde-inducible promoter for dynamically regulated Escherichia coli growth on methanol.[J]. Acs Synthetic Biology, 2017, 6(8).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]