Difference between revisions of "Part:BBa K2586001"
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K2586001 short</partinfo> | <partinfo>BBa_K2586001 short</partinfo> | ||
| + | |||
This part is coding for a glutamate and glyphosate transporter. | This part is coding for a glutamate and glyphosate transporter. | ||
| Line 7: | Line 8: | ||
Bacteria lacking the <i>gltT</i> gene are highly resistant to glyphosate because the herbicide is not transported into the cell. This part could be useful to engineer bacteria for the uptake and degradation of the weedkiller. | Bacteria lacking the <i>gltT</i> gene are highly resistant to glyphosate because the herbicide is not transported into the cell. This part could be useful to engineer bacteria for the uptake and degradation of the weedkiller. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 31: | Line 21: | ||
<partinfo>BBa_K2586001 parameters</partinfo> | <partinfo>BBa_K2586001 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | ==Characterization== | ||
| + | |||
| + | <b>Deletion of <i>gltT</i> confers glyphosate tolerance</b> | ||
| + | |||
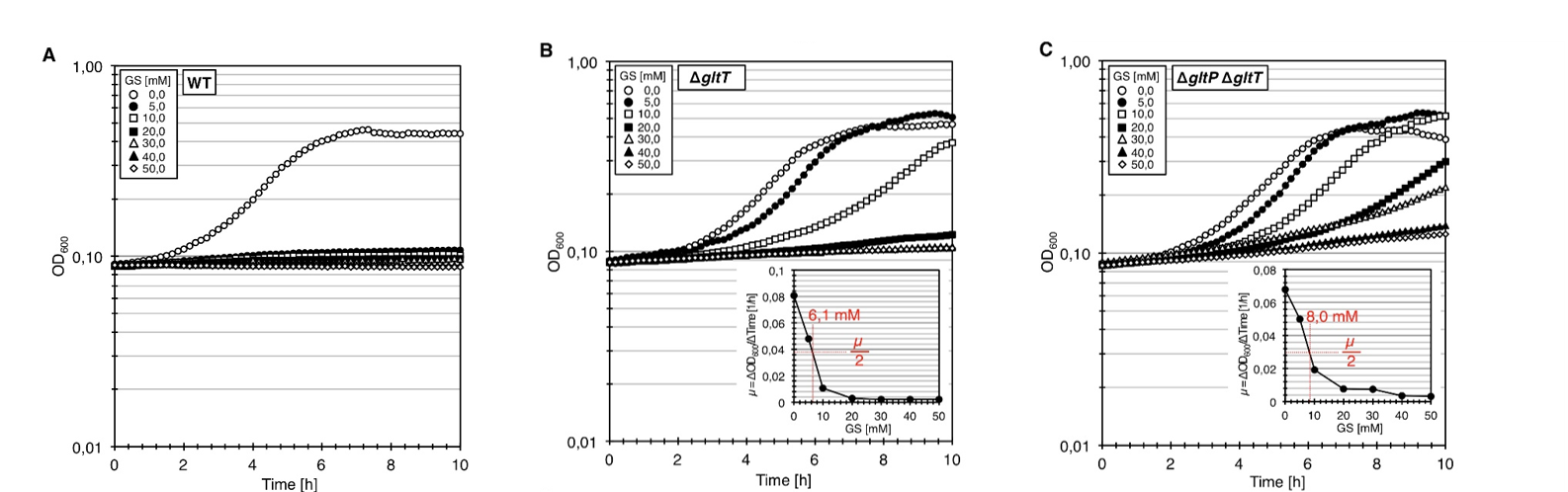
| + | We tested whether the clean deletion of the <i>gltT</i> gene sufficient to confer high-level resistance of B. subtilis to glyphosate. For this purpose, we constructed the mutant strain BP233 (<i>gltT</i>). To assess the glyphosate resistance of the <i>gltT</i> mutant, we cultivated the bacteria in CS-Glc minimal medium supplemented with increasing amounts of glyphosate. As shown in Figure 1A, growth of the wild type was inhibited by 5 mM glyphosate. The transporter with a high-affinity for glyphosate seems to be in fact GltT because the deletion of the gltT gene conferred high-level resistance to the herbicide (Figure 1C). Moreover, the growth rates of the strain BP233 (<i>gltT</i>) were reduced by 50% at herbicide concentrations of 6.1 mM (Figure 1B). Thus, in comparison to the wild type strain, 6-fold higher glyphosate concentration is needed to reduce the growth rate of the strain BP233 (<i>gltT</i>) by 50%. | ||
| + | |||
| + | |||
| + | <b>Double mutant <i>gltT gltP</i> shows even higher glyphosate tolerance </b> | ||
| + | |||
| + | In order to test whether the deletion of both gltT and gltP confers an even higher glyphosate tolerance, we constructed the mutant strain BP235 (<i>gltT gltP</i>). We cultivated the bacteria in CS-Glc minimal medium supplemented with increasing amounts of glyphosate. As shown in Figure 1D, the double deletion increased the glyphosate tolerance to 8.0 mM. Therefore, an 8-fold higher glyphosate concentration is needed to reduce the growth rate of the strain BP235 (<i>gltT gltP</i>) by 50%. | ||
| + | |||
| + | |||
| + | [[File:T--Goettingen--BBa_K2586001_platereader.png|900px|center|thumb|'''Fig. 1.''' <b>Inactivation of the glutamate transporters confers high-level resistance to glyphosate.</b> (A) Growth of the B. subtilis wild type (WT) strain 168 in CS-Glc minimal medium supplemented with increasing amounts of glyphosate (GS). (B) Growth of the Δ(<i>gltT</i>) mutant strain BP233 in CS-Glc minimal medium supplemented with increasing amounts of glyphosate (GS). The figure inlay shows the relationship between the growth rate (µ) and the glyphosate (GS) concentration.(C) Growth of the Δ(<i>gltT</i>) Δ(<i>gltP</i>) mutant strain BP235 in CS-Glc minimal medium supplemented with increasing amounts of glyphosate (GS). The figure inlay shows the relationship between the growth rate (µ) and the glyphosate (GS) concentration.]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | [[File:T--goettingen--Roundup gltT gltP.jpg|400px|thumb|'''Fig. 2.''' <b>A. </b>Growth of the strains 168 (wild type ), BP233 (Δ<i>gltT</i>), BP234 (Δ<i>gltP</i>) and BP235 (Δ<i>gltT</i> Δ<i>gltP</i>) on CS-Glc minimal medium in the presence of different amounts of glyphosate. B. Roundup® Alphee purchased from Westfalia. C. Growth of the strains 168, BP233, BP234 and BP235 on CS-Glc minimal medium in the presence of different amounts of Roundup® Alphee.]] | ||
| + | |||
| + | <b>The deletion strains show also high resistance to commercially available RoundUp</b> | ||
| + | |||
| + | |||
| + | We also tested whether the glyphosate that is present in commercially available Roundup® is toxic for <i>B. subtilis</i>. Roundup® was ordered from the german company [https://www.westfalia.de/shops/garten/pflanzenschutz_und_duenger/unkrautbekaempfung/pflanzenschutzmittel/1228751-unkrautfrei_alphee_1_liter_die_nr_1_gegen_unkraut.htm? Westfalia]. 1 l of Roundup® Alphee contains 9.4 g of the isopropylamine salt of glyphosate (which corresponds to 7.2 g/l pure glyphosate), 5 g surfactant (undefined) and water. To test the effect of Roundup® Alphee on growth of the bacteria, we propagated the <i>B. subtilis</i> strains 168 (wild type ), BP233 (Δ<i>gltT</i>), BP234 (Δ<i>gltP</i>) and BP235 (Δ<i>gltT</i> Δ<i>gltP</i>) on CS-Glc minimal medium agar plates that were supplemented with glyphosate (control) and with equimolar amounts of glyphosate present in Roundup (test conditions). As shown in Figure 9, Roundup® Alphee killed the <i>B. subtilis</i> wild type strain and the strain lacking the low-affinity glutamate transporter GltP already at a concentration of 2 mM. By contrast, all strains lacking the high-affinity transporter GltT grew in the presence of 2 - 6 mM glyphosate that is present in Roundup. | ||
| + | |||
| + | <b>Complementation of the gltT mutation</b> | ||
| + | |||
| + | We also performed a complementation experiment to provide further evidence that GltT is the major glyphosate transporter in <i>B. subtilis</i>. For this purpose, we fused the artificial Palf4 promoter [https://parts.igem.org/Part:BBa_K2586000 BBa_K2586000] together with the gapA ribosome-binding site [https://parts.igem.org/Part:BBa_K2586008 BBa_K2586008] to the gltT gene and integrated the construct into the amyE locus of the gltT mutant (Figure 3A). Next, we propagated the strains BP233 (gltT) and BP237 (gltT Palf4-gltT) together with the wild type strain 168 on agar plates without and with glyphosate (Figure 3B). As expected, all strains grew in the absence of glyphosate. By contrast, only the gltT mutant strain BP233 grew with glyphosate. Thus, like the wild type also the complementation strain BP237 take up glyphosate via GltT. To conclude, under the tested growth conditions the high-affinity glutamate transporter GltT is the major entryway of glyphosate into <i>B. subtilis</i>!. | ||
Revision as of 11:22, 22 September 2018
GltT: glutamate and glyphosate transporter in B. subtilis
This part is coding for a glutamate and glyphosate transporter.
The DNA sequence of the gltT gene is coding for the GltT transporter, which is responsible for glutamate uptake into the cell. We found that GltT is also involved in glyphosate uptake in the Gram-positive model bacterium Bacillus subtilis.
Bacteria lacking the gltT gene are highly resistant to glyphosate because the herbicide is not transported into the cell. This part could be useful to engineer bacteria for the uptake and degradation of the weedkiller.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 742
Illegal NgoMIV site found at 1123 - 1000COMPATIBLE WITH RFC[1000]
Characterization
Deletion of gltT confers glyphosate tolerance
We tested whether the clean deletion of the gltT gene sufficient to confer high-level resistance of B. subtilis to glyphosate. For this purpose, we constructed the mutant strain BP233 (gltT). To assess the glyphosate resistance of the gltT mutant, we cultivated the bacteria in CS-Glc minimal medium supplemented with increasing amounts of glyphosate. As shown in Figure 1A, growth of the wild type was inhibited by 5 mM glyphosate. The transporter with a high-affinity for glyphosate seems to be in fact GltT because the deletion of the gltT gene conferred high-level resistance to the herbicide (Figure 1C). Moreover, the growth rates of the strain BP233 (gltT) were reduced by 50% at herbicide concentrations of 6.1 mM (Figure 1B). Thus, in comparison to the wild type strain, 6-fold higher glyphosate concentration is needed to reduce the growth rate of the strain BP233 (gltT) by 50%.
Double mutant gltT gltP shows even higher glyphosate tolerance
In order to test whether the deletion of both gltT and gltP confers an even higher glyphosate tolerance, we constructed the mutant strain BP235 (gltT gltP). We cultivated the bacteria in CS-Glc minimal medium supplemented with increasing amounts of glyphosate. As shown in Figure 1D, the double deletion increased the glyphosate tolerance to 8.0 mM. Therefore, an 8-fold higher glyphosate concentration is needed to reduce the growth rate of the strain BP235 (gltT gltP) by 50%.
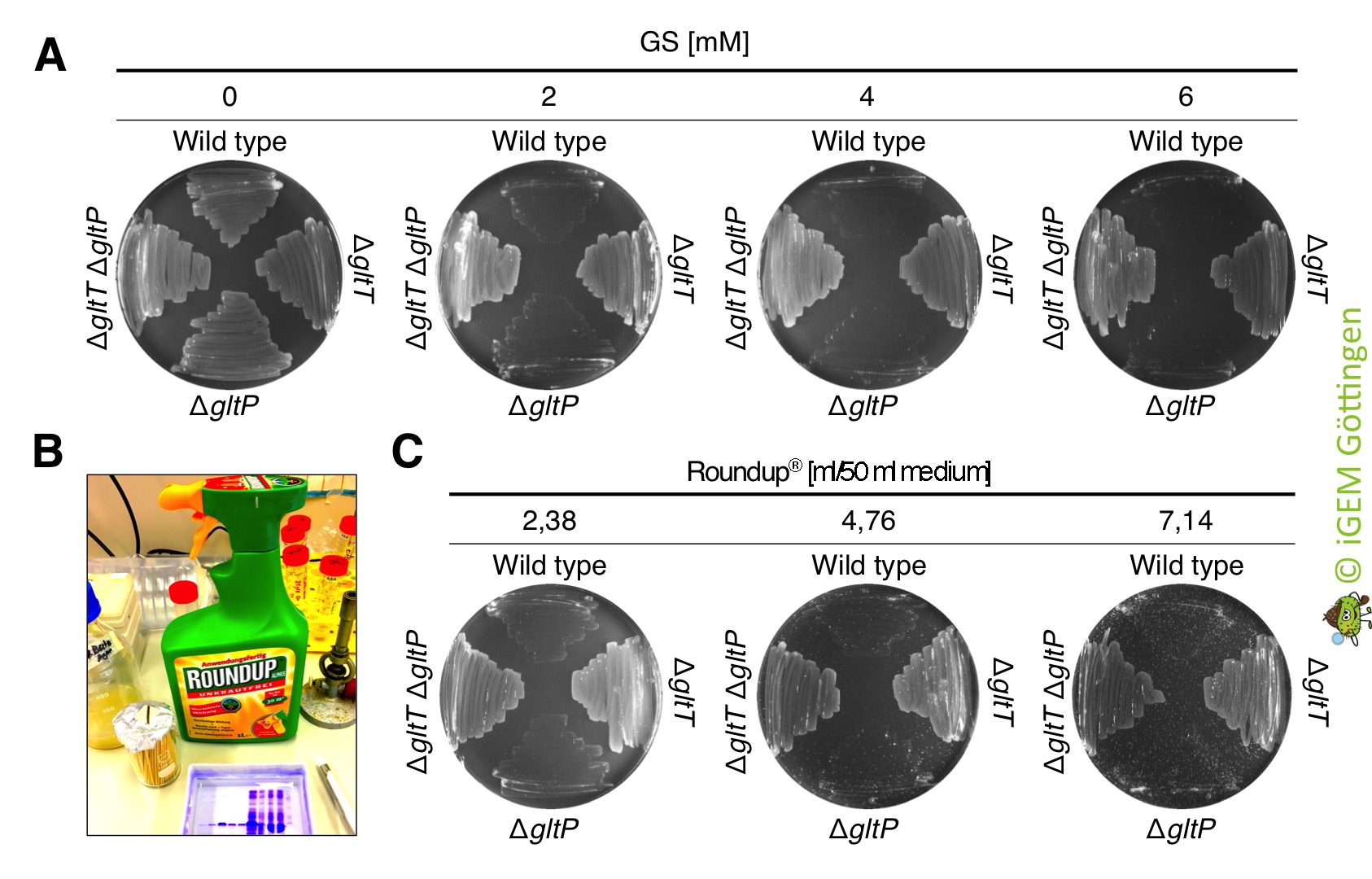
The deletion strains show also high resistance to commercially available RoundUp
We also tested whether the glyphosate that is present in commercially available Roundup® is toxic for B. subtilis. Roundup® was ordered from the german company Westfalia. 1 l of Roundup® Alphee contains 9.4 g of the isopropylamine salt of glyphosate (which corresponds to 7.2 g/l pure glyphosate), 5 g surfactant (undefined) and water. To test the effect of Roundup® Alphee on growth of the bacteria, we propagated the B. subtilis strains 168 (wild type ), BP233 (ΔgltT), BP234 (ΔgltP) and BP235 (ΔgltT ΔgltP) on CS-Glc minimal medium agar plates that were supplemented with glyphosate (control) and with equimolar amounts of glyphosate present in Roundup (test conditions). As shown in Figure 9, Roundup® Alphee killed the B. subtilis wild type strain and the strain lacking the low-affinity glutamate transporter GltP already at a concentration of 2 mM. By contrast, all strains lacking the high-affinity transporter GltT grew in the presence of 2 - 6 mM glyphosate that is present in Roundup.
Complementation of the gltT mutation
We also performed a complementation experiment to provide further evidence that GltT is the major glyphosate transporter in B. subtilis. For this purpose, we fused the artificial Palf4 promoter BBa_K2586000 together with the gapA ribosome-binding site BBa_K2586008 to the gltT gene and integrated the construct into the amyE locus of the gltT mutant (Figure 3A). Next, we propagated the strains BP233 (gltT) and BP237 (gltT Palf4-gltT) together with the wild type strain 168 on agar plates without and with glyphosate (Figure 3B). As expected, all strains grew in the absence of glyphosate. By contrast, only the gltT mutant strain BP233 grew with glyphosate. Thus, like the wild type also the complementation strain BP237 take up glyphosate via GltT. To conclude, under the tested growth conditions the high-affinity glutamate transporter GltT is the major entryway of glyphosate into B. subtilis!.
