Difference between revisions of "Part:BBa K2455003"
(→Purification and SDS-PAGE) |
(→Purification and SDS-PAGE) |
||
| Line 84: | Line 84: | ||
Following correct insertion of the [[Part:BBa_K864100|SYFP2]] and [[Part:BBa_K592100|mTag BFP]] biobricks into the [[Part:BBa_K863006|Cell-Penetrating USER Cassette]], expression was done in <i>Escherichia coli</i> BL21 under control of the T7 promoter. | Following correct insertion of the [[Part:BBa_K864100|SYFP2]] and [[Part:BBa_K592100|mTag BFP]] biobricks into the [[Part:BBa_K863006|Cell-Penetrating USER Cassette]], expression was done in <i>Escherichia coli</i> BL21 under control of the T7 promoter. | ||
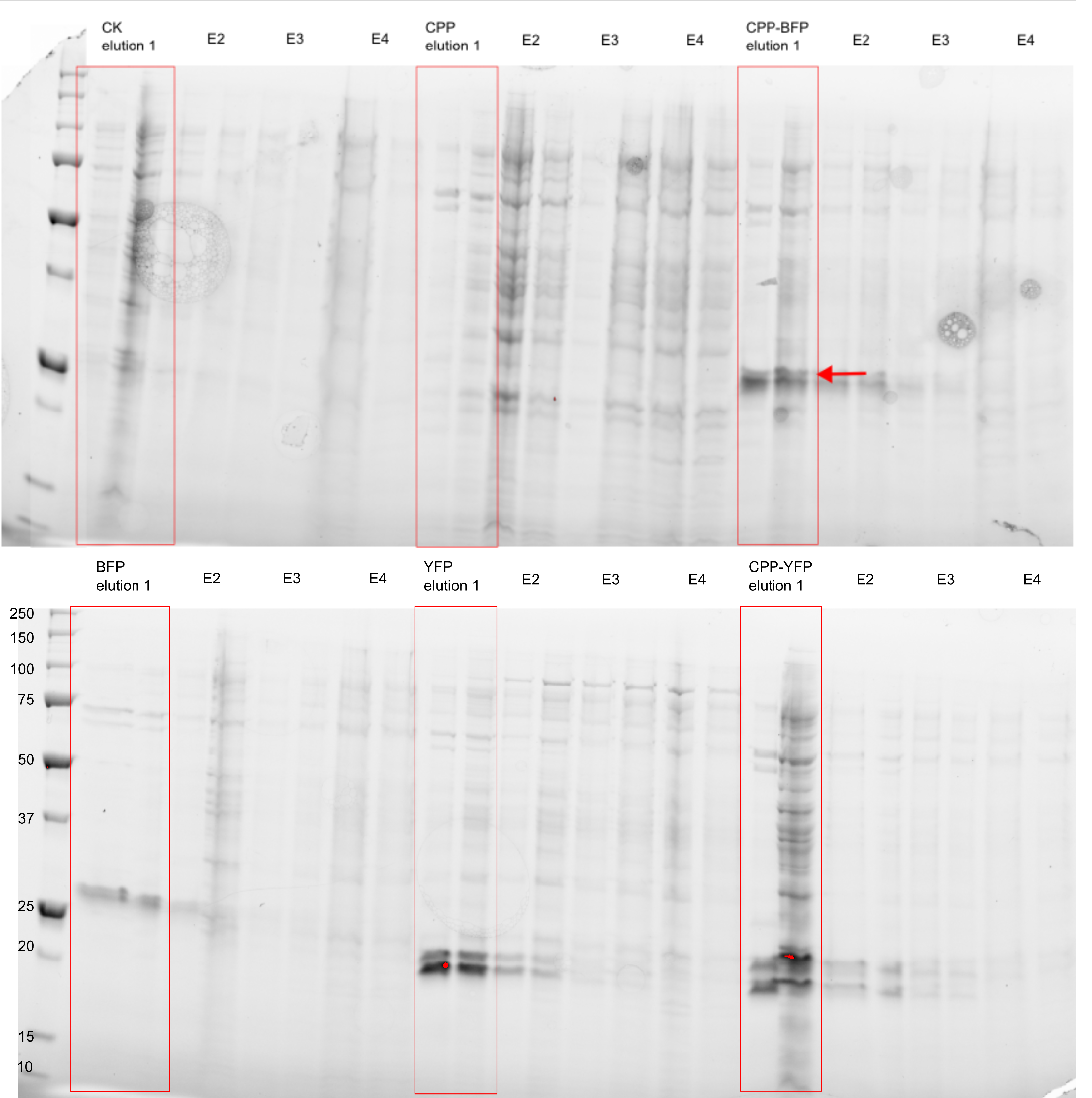
| − | [[Image: My_western_image.png| | + | [[Image: My_western_image.png|300px|thumb|right|'''Figure 3: Purification of fluorescent proteins.''' Expressed proteins were purified using an Imidazole gradient; E1: 250 mM, E2: 500 mM, E3: 1.0 M, E4: 2.0 M]] |
===Purification=== | ===Purification=== | ||
| Line 92: | Line 92: | ||
===SDS-PAGE=== | ===SDS-PAGE=== | ||
| − | The purity of the samples was analysed by subjecting them to an SDS-PAGE using a [http://www.bio-rad.com/en-cn/product/criterion-precast-gels/criterion-tgx-stain-free-precast-gels pre-cast stain free gel]. In addition to the CPP tagged version of [[Part:BBa_K864100|SYFP2]] and [[Part:BBa_K592100|mTag BFP]] two versions without the CPP tag was also purified, as well as an empty vector and a vector with an empty [[Part:BBa_K863006|Cell-Penetrating USER Cassette]]. The Imidazole concentration of the purification ranged from 250 mM to 2.0 M, as can be seen on Figure 3 proteins of the expected size are eluted at Imidazole concentrations of 250 mM for all constructs (BFP & YFP 28kDa, CPP-BFP & CPP-YFP 30kDa). | + | The purity of the samples was analysed by subjecting them to an SDS-PAGE using a [http://www.bio-rad.com/en-cn/product/criterion-precast-gels/criterion-tgx-stain-free-precast-gels pre-cast stain free gel]. In addition to the CPP tagged version of [[Part:BBa_K864100|SYFP2]] and [[Part:BBa_K592100|mTag BFP]] two versions without the CPP tag was also purified, as well as an empty vector and a vector with an empty [[Part:BBa_K863006|Cell-Penetrating USER Cassette]]. The Imidazole concentration of the purification ranged from 250 mM to 2.0 M, as can be seen on Figure 3 proteins of the expected size are eluted at Imidazole concentrations of 250 mM for all constructs (BFP & YFP 28kDa, CPP-BFP & CPP-YFP 30kDa). |
==Imaging== | ==Imaging== | ||
Revision as of 23:04, 1 November 2017
Cell-Penetrating USER Cassette
This biobrick contains an AsiSI/Nb.BSMI USER Cassette with a N-terminal nona-arginine (R9) Cell-penetrating peptide which gives proteins inserted into the cassette the ability to pass through plasma membranes. In addition the USER Cassette carries a N-terminal hexa-histidine tag which allows for easy purification.
For our study we demonstrated that proteins inserted into the cassette can be purified using the histidine-tag, and subsequently used to stain E. coli and Pseudomonas putidacells, presumably through internalization cause by the R9 peptide.
In addition; previous studies have shown proteins associated with R9, either covalently or non-covalently, to be able to enter a variety of cell types (Chang et al.).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Cell-Penetrating Peptides (CPPs) are small peptides, typically rich in basic residues, which are able to facilitate transport of a wide variety of cargoes across plasma membranes. Their origin in nature comes from viral domains such as the viral HIV tat domain (Eudes & Chugh, 2008). In recent years research have been done into making synthetic CPPs, especially peptides constructed solely from arginine residues have been of interest. The arginine rich sequence has been shown to trigger endocytosis in a wide range of cell types, including onion and potato cells (Chang et al.).
USER Cassette
The biobrick carries the AsiSI/Nb.BsmI USER Cassette which facilitates gene insertion through the use of USER cloning.

For more information on USER cloning see [http://www.cbs.dtu.dk/services/AMUSER/ Amuser] or [http://www.cbs.dtu.dk/services/AMUSER/instructions.php Amuser instructions].
Insertion Requirements
For insertion of genes into the USER Cassette genes have to be amplified with the primers carrying the two following overhangs:
Forward primer: CGTGCGAUCx
Reverse primer: CACGCGAUxx
Where U = Uracil and x = arbitrary nucleotide; these are needed to keep the inserted gene in-frame. AsiSI and Nb.BsmI have to be used to facilitate opening of the cassette.
Gene Insertion
In order to test the ability of the biobrick to facilitate protein import in E. coli we choose to insert two different fluorescent proteins into it, SYFP2 and mTag BFP.
Opening of USER Cassette
To facilitate insertion we first had to prepare the vector, this was done in a two step digestion reaction. First to open the cassette 42 µL purified vector was mixed with 2.5 µL AsiSI, 10 µL 10x Tango Buffer, and 45.5 µL Nuclease-free water. The mixture was then incubated for 3 hours at 37 degrees Celsius. 5 µL linearised vector was run for 20 min at 100 V on a 1 % Agarose gel to check for proper linearisation. As can be seen on Figure 2 the vector goes from a smaller band, typical for linearised (supercoiled) pieces of DNA, while the digested vector has a band consistent with the full 6kbp of the vector.
Following digestion the vector was subjected to column-purified using [http://omegabiotek.com/store/product/e-z-n-a-gel-extraction-kit/ E.Z.N.A.® Gel Extraction Kit] and [http://omegabiotek.com/store/wp-content/uploads/2013/05/D2500.D2501-Gel-Extraction-Kit-Combo-Online.pdf standard protocol] for gel-extraction with 150 uL binding buffer being added in the first step.
Finally the vector was nicked by mixing the complete 50 uL elute from the column-purification with 1 uL Nb.BsmI, 6 uL 10x NEBuffer 3.1, and 3 uL Nuclease-free water. The mixture was incubated for 3 hours at 65 degrees Celcius.
Gene Amplification
In order to facilitate insertion of the SYFP2 and mTag BFP biobricks into the USER Cassette, the two genes were amplified using the following sets of primers in order to add the appropriate overhangs:
SYFP2:
Forward primer: CGTGCGAUCA-ATGGTTAGCAAGGGCGAAG
Reverse primer: CACGCGAUGA-TTTATACAGCTCATCCATACCCAGGG
mTag BFP:
Forward primer: CGTGCGAUCA-ATGAGCGAACTGATCAAAGAG
Reverse primer: CACGCGAUGA-ATTCAGTTTATGACCCAGC
And gel-extracted using [http://omegabiotek.com/store/product/e-z-n-a-gel-extraction-kit/ E.Z.N.A.® Gel Extraction Kit] and [http://omegabiotek.com/store/wp-content/uploads/2013/05/D2500.D2501-Gel-Extraction-Kit-Combo-Online.pdf standard protocol].
Insertion of BFP and YFP
Insertion of the gel-extracted PCR products was done by mixing 1 µL PCR product with 2 µL linearised vector, 0.5 µL CutSmart® Buffer, 0.5 µL USER® Enzyme, and 1 µL MQ H2O. The mixture was incubated in a PCR machine for 25 min at 37 degrees Celcius, 20 min at 25 degrees Celcius, 10 min at 20 degrees Celcius, and 10 min at 15 degrees Celcius.
Correct insertion was validated through PCR amplification of inserts and subsequent sequencing.
Purification and SDS-PAGE
Following correct insertion of the SYFP2 and mTag BFP biobricks into the Cell-Penetrating USER Cassette, expression was done in Escherichia coli BL21 under control of the T7 promoter.
Purification
Cells were harvested, re-suspended in a lysis buffer containing 25 mM Imidazole, 0.5 M NaCl, 20 mM Tris-HCl (pH 7.9), and 1.0 mM PMSF, and lysed by sonication. Cell debris was removed by centrifugation at low speed, and the supernatant was transferred to a fresh falcon tube and mixed with 100 µL Ni-NTA Agarose. The mixture was incubated for one hour on a cold room turning table, where after the beads were spun down and the supernatant removed. A series of elution buffers (0.5 M NaCl, 20 mM Tris-HCl; pH 7.9) with increasing Imidazole concentration was used to elute the bound proteins by consecutively adding 100 µL elution buffer, centrifuging the sample at low speed, and removing 100 µL eluate.
NOTE: Samples were kept on ice at all times and centrifugation took place at 4 degrees Celsius.
SDS-PAGE
The purity of the samples was analysed by subjecting them to an SDS-PAGE using a [http://www.bio-rad.com/en-cn/product/criterion-precast-gels/criterion-tgx-stain-free-precast-gels pre-cast stain free gel]. In addition to the CPP tagged version of SYFP2 and mTag BFP two versions without the CPP tag was also purified, as well as an empty vector and a vector with an empty Cell-Penetrating USER Cassette. The Imidazole concentration of the purification ranged from 250 mM to 2.0 M, as can be seen on Figure 3 proteins of the expected size are eluted at Imidazole concentrations of 250 mM for all constructs (BFP & YFP 28kDa, CPP-BFP & CPP-YFP 30kDa).
Imaging
All fluorescent imaging was done at 100x magnification using the following setup:
Yellow fluorescence:
- Excitation time: 250 ms
- Excitation: 460 - 490 nm
- Dicroitic Mirror: 505 nm
- Barrier Filter: 510 - 555 nm
Blue fluorescence:
- Excitation time: 100 ms
- Excitation: 330 - 385 nm
- Dicroitic Mirror: 400 nm
- Barrier Filter: >420 nm
Fluorescent Imaging of Expressing Cells
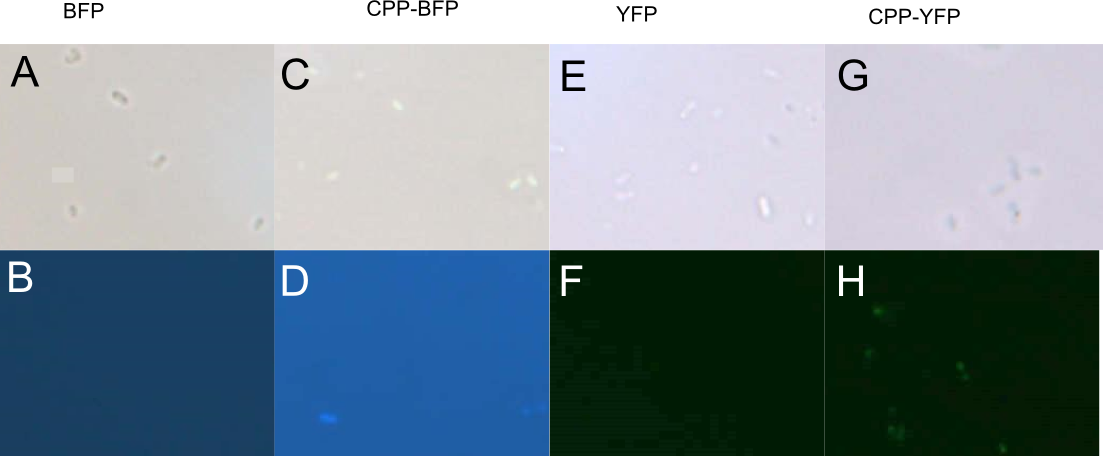
BL 21 E. coli expressing either SYFP2, mTag BFP, CPP-SYFP2, and CPP-mTag BFP were analysed on a fluorescent microscope at 100x magnification in order to see if the fusion proteins were capable of emitting fluorescent light as their parental counterparts. As can be see on Figure 4 the intensity of the individual cells were as bright with the CPP tag as without.
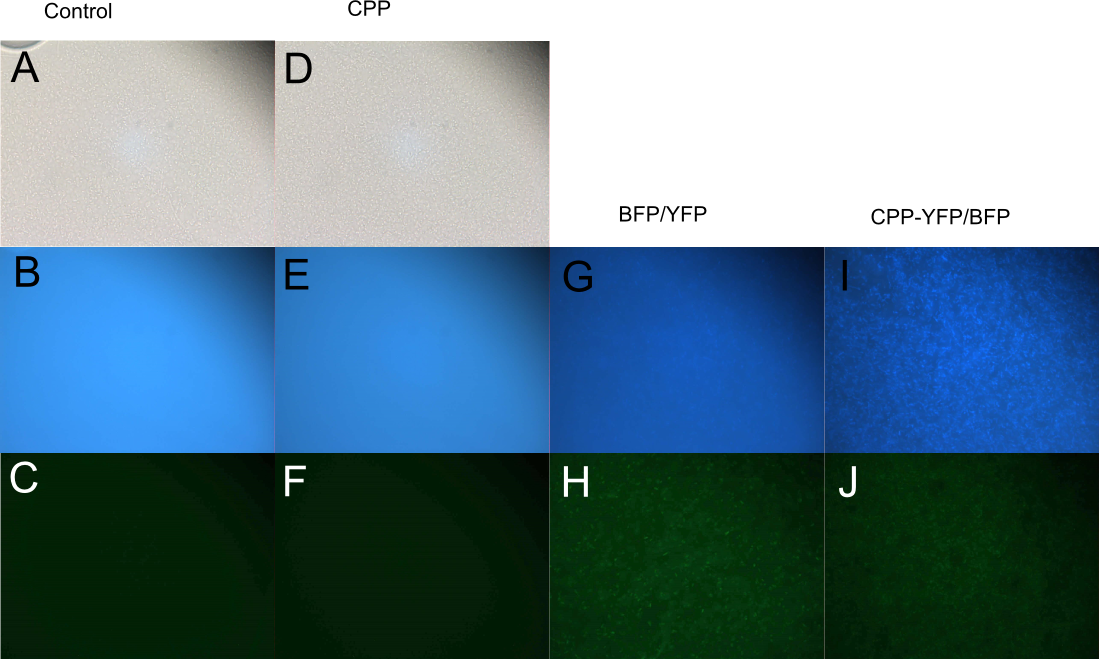
Cell-Penetrating Capabilities of Purified Proteins
In order to investigate the capabilities of expressed proteins to enter E. coli cells, 10 µL of E. coli MG1655 overnight culture was mixed with 25 µL purified protein from either Control (empty vector), SYFP2, mTag BFP, CPP, CPP-SYFP2, or CPP-mTag BFP and incubated at room temperature for 10 min. Subsequently the cells were pelleted and the supernatant was removed, followed by 2 x wash in 500 mL MQ H2O. Finally the cells were re-suspended in 10 µL MQ H2O before being imaged on the fluorescent microscope. As can be seen on Figure 5, while neither SYFP2 or mTag BFP alone were capable of staining the cells, both CPP-SYFP2 and CPP-mTag BFP were able too, thus indicating an ability of the Cell-Penetrating USER Cassette to facilitate internalization of their protein cargo.
In addition to E. coli, both Pseudomonas putida and Bacillus subtilis were similarly treated with SYFP2, mTag BFP, CPP-SYFP2, or CPP-mTag BFP following the same protocol as for E. coli (see above). While B. subtilis showed no signs of protein uptake (data not shown), P. putida showed similar signs to protein uptake as did E. coli (see Fig. 6).