Difference between revisions of "Part:BBa K2273041"
| Line 28: | Line 28: | ||
A signal peptide ([https://parts.igem.org/Part:BBa_K2273023 AmyE SP]) was fused n-terminally to this construct to induce secretion. | A signal peptide ([https://parts.igem.org/Part:BBa_K2273023 AmyE SP]) was fused n-terminally to this construct to induce secretion. | ||
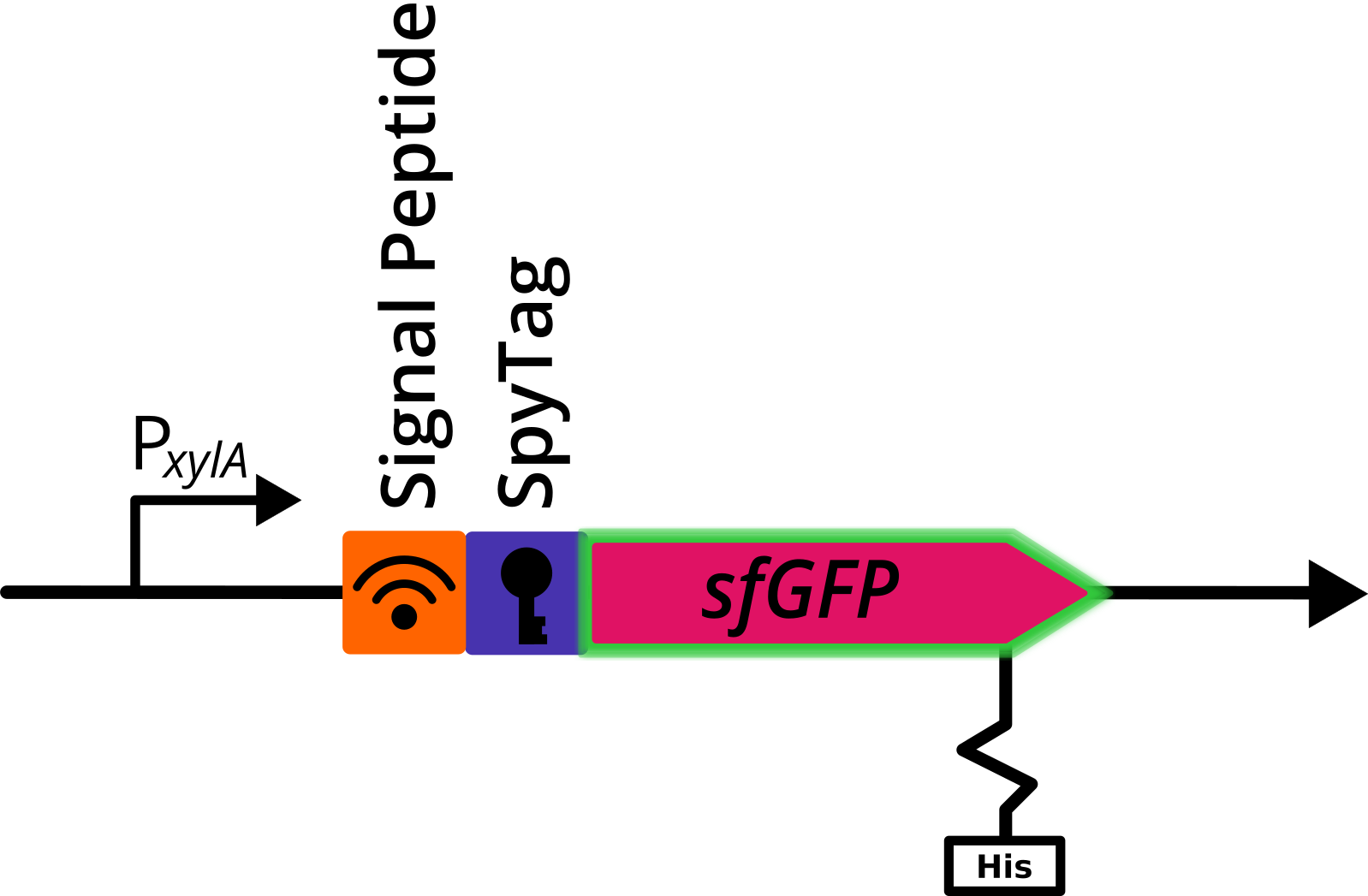
| − | [[File:Tag gfp.png|thumb|centre|600px|''' Figure | + | [[File:Tag gfp.png|thumb|centre|600px|''' Figure 1:''' Genetic construct with sfGFP. Depicted is a translational fusion constructs downstream of the <i>PxylA</i> promotor, that were cloned in the multiple cloning site of the pBS2E<i><sub>PxylA</sub></i> vector. The construct contains a signal peptide sequence, the gene coding for sfGFP, n-terminally fused SpyTag and a his-tag.]] |
| Line 42: | Line 42: | ||
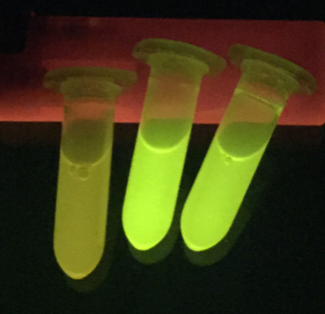
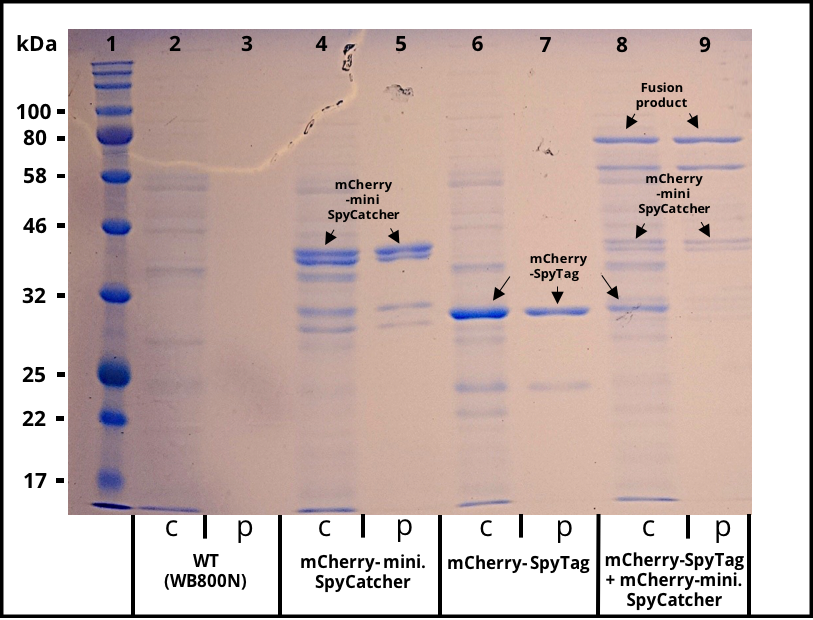
The successful secretion could be proven with a fluorescence assay using the supernatants of <i>B. subtilis </i> (Figure 2 and Figure 3). The functionality of the SpyTag/SpyCatcher was proven via SDS-PAGE, using the supernatants (Figure 4). | The successful secretion could be proven with a fluorescence assay using the supernatants of <i>B. subtilis </i> (Figure 2 and Figure 3). The functionality of the SpyTag/SpyCatcher was proven via SDS-PAGE, using the supernatants (Figure 4). | ||
| − | [[File:T--TU Dresden--secretion--Figure1.png|thumb|right|500px|''' Figure | + | [[File:T--TU Dresden--secretion--Figure1.png|thumb|right|500px|''' Figure 2:''' Endpoint measurement of the fluorescence from supernatants carrying our constructs and the wild type. Expression of the single copy mCherry or sfGFP fusion SpyTag/SpyCather constructs (purple) was induced with 1% xylose and the supernatants were harvested after 16 h of incubation. Wild type supernatant is shown as a control (pink). Excitation wavelength for sfGFP was set to 480 nm and emission was recorded at 510 nm and for mCherry excitation wavelength was set to 585 nm and emission was recorded at 615 nm . The fluorescence was normalized by the optical density (OD600). Graph shows mean values and standard deviations of at least two biological and three technical replicates.]] |
| − | [[File:T--TU Dresden--secretion--supernatantsfGFP.png|thumb|left|700px|''' Figure | + | [[File:T--TU Dresden--secretion--supernatantsfGFP.png|thumb|left|700px|''' Figure 3:''' Supernatants of B. subtilis cultures excited with blue light. Wild-type supernatant (left) and a SpyTag-sfGFP secreting strain (middle and right). The expression of the multi-copy sfGFP was induced with 1% Xylose and the supernatant was harvested after 16 h of incubation. The fluorescence was induced with a “Dark Reader Transilluminator”.]] |
Revision as of 18:59, 1 November 2017
| Part Information | |
|---|---|
| RFC standard | RFC 25 |
| Fused Tag | BBa_K2273014: SpyTag |
| Fluorescent Protein | BBa_K2273021: sfGFP His-tagged |
| Submitted by | [http://2017.igem.org/Team:TU_Dresden TU Dresden] |
sfGFP with N-Terminal SpyTag and C-Terminal His Tag
This composite part was used for evalutaion in the [http://2017.igem.org/Team:TU_Dresden/Project/Secretion secretion project] of 2017 TU_Dresden iGEM [http://2017.igem.org/Team:TU_Dresden team]. It codes for a fluorescent reporter protein (sfGFP) and a functional tag (SpyTag), mediating covalent bonding with it tag partner (SpyCatcher). It is optimized for usage in Bacillus subtilis.
Design
A signal peptide (AmyE SP) was fused n-terminally to this construct to induce secretion.
Application
The successful secretion could be proven with a fluorescence assay using the supernatants of B. subtilis (Figure 2 and Figure 3). The functionality of the SpyTag/SpyCatcher was proven via SDS-PAGE, using the supernatants (Figure 4).
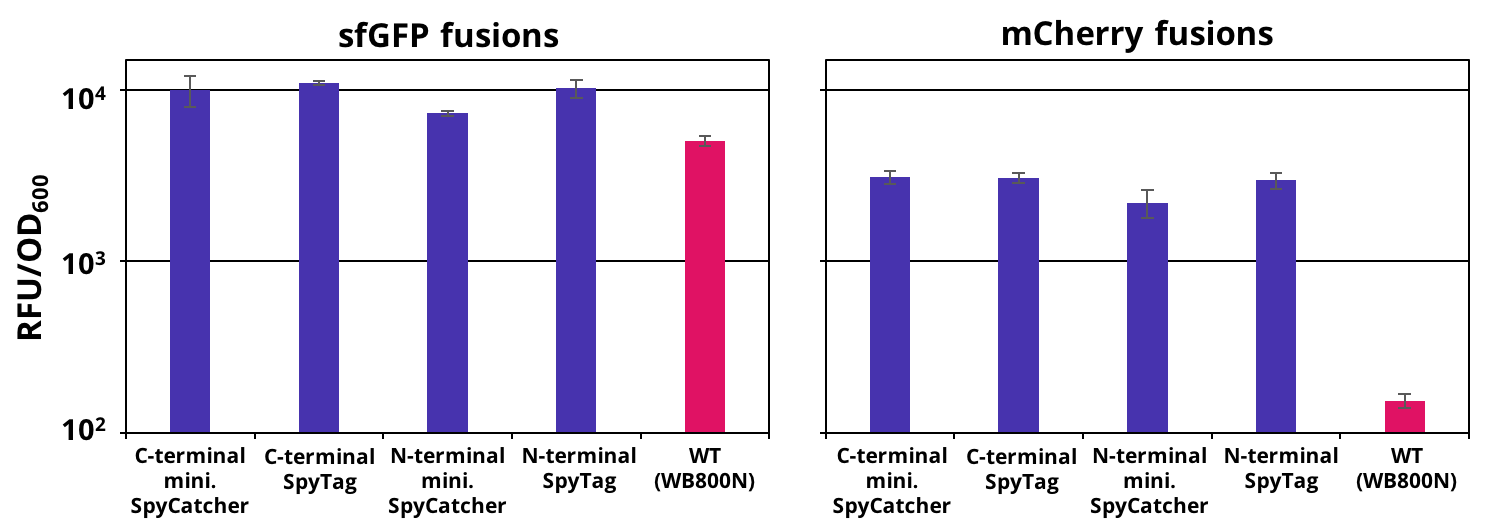
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
