Difference between revisions of "Part:BBa J04450"
| Line 46: | Line 46: | ||
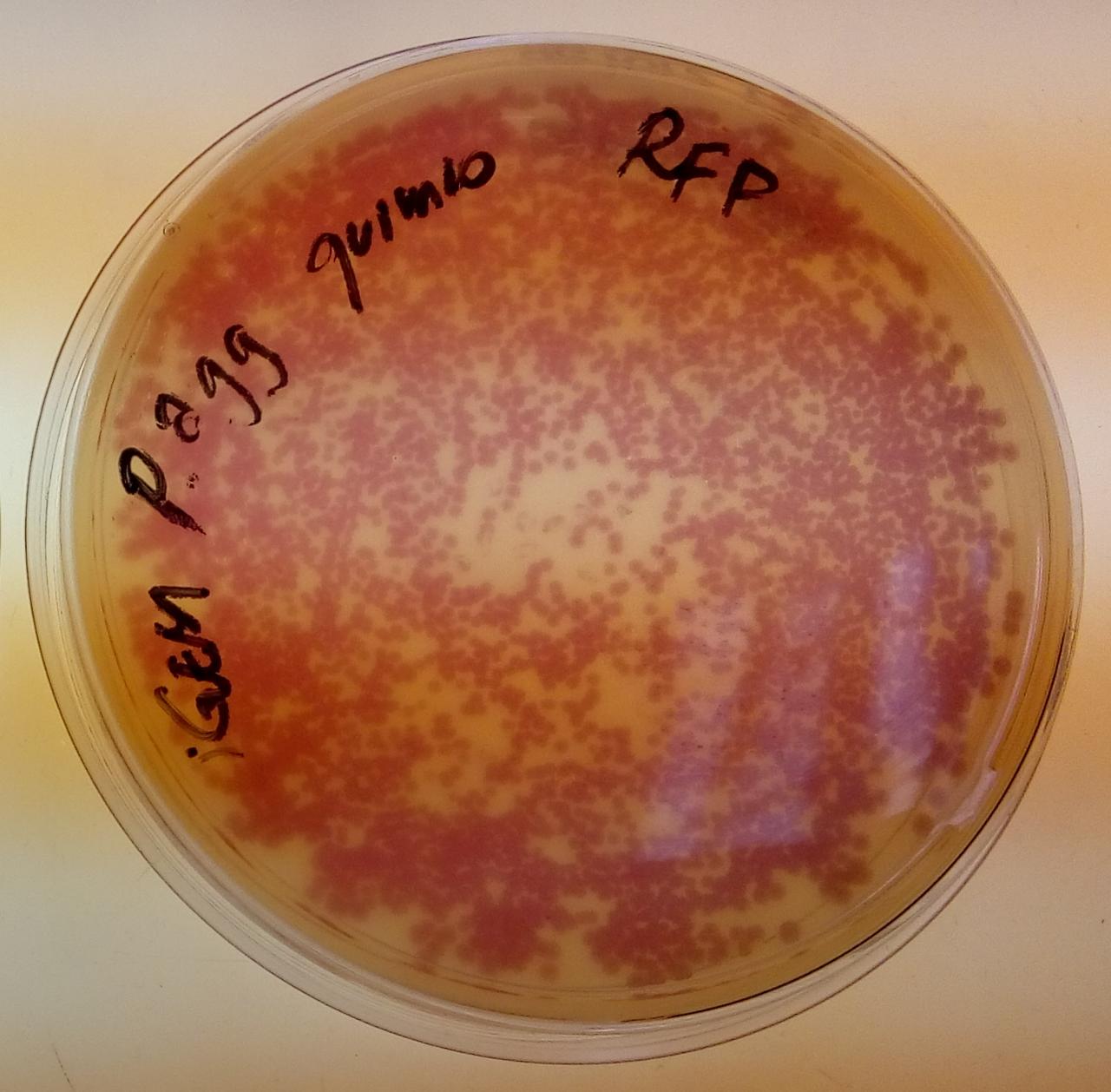
Image:T--USP-Brazil--Registry--RFP-pagglomerans.jpeg|''Pantoea agglomerans'' colonies in chloramphenicol, containing BBa_J04450 in the pSB1C3 backbone | Image:T--USP-Brazil--Registry--RFP-pagglomerans.jpeg|''Pantoea agglomerans'' colonies in chloramphenicol, containing BBa_J04450 in the pSB1C3 backbone | ||
</gallery> | </gallery> | ||
| + | |||
| + | ==Team ITB_Indonesia 2017: Red color dynamics of cloned <i>Escherichia coli</i> strains in LB broth== | ||
| + | |||
| + | <html> | ||
| + | |||
| + | <p> | ||
| + | <figure> | ||
| + | <img src="https://static.igem.org/mediawiki/parts/5/53/Barenganstrain.JPG" alt="Three strains expression on mRFP" style="float:right;width:350px;height:205.5px;"> | ||
| + | |||
| + | </figure> | ||
| + | |||
| + | <i>Background</i><br><br> | ||
| + | |||
| + | In <a href="http://2017.igem.org/Team:ITB_Indonesia" target="_blank">Team ITB_Indonesia 2017</a> | ||
| + | |||
| + | characterization, we found in normal growth/incubation condition (37 <sup>o</sup>C, LB agar) that | ||
| + | |||
| + | BBa_J04450-transformed <i>Escherichia coli</i> BL21 colony appear to need longer incubation time (>18 | ||
| + | |||
| + | hours) until it clearly shows red color under natural light.<br><br> | ||
| + | |||
| + | We then investigate whether this phenomenon is influenced by the strain, and we try if there are | ||
| + | |||
| + | <i>lac</i> repressor in the system that can be released by inducing the culture with IPTG, hence | ||
| + | |||
| + | increasing the expression of mRFP.<br><br> | ||
| + | |||
| + | <i>Experimental Design</i><br><br> | ||
| + | |||
| + | We used three different strains of transformed <i>E. coli</i> (BL21, DH5alpha, and Top10) for this | ||
| + | |||
| + | study. They were incubated in LB broth, 37 <sup>o</sup>C, and sampled every 4 hours for 2 days to | ||
| + | |||
| + | determine the red color absorbance at 588 nm. The amount of IPTG added for respective treatment is 500 | ||
| + | |||
| + | µM.<br><br> | ||
| + | |||
| + | <i>Result and Findings</i><br> | ||
| + | <ul> | ||
| + | <li>There are no significant differences of mRFP expression in different strains of <i>E. coli</i> | ||
| + | |||
| + | (BL21, DH5alpha, Top10)</li> | ||
| + | <li>There are no significant effects of mRFP increased expression after IPTG induction.</li> | ||
| + | <li>The red color absorbance under 588 nm wavelength is recorded around 2.5-3 OD units.</li> | ||
| + | <li>The broth become red in color under natural light around 16-20 hours of incubation time.</li> | ||
| + | </ul> | ||
| + | <br> | ||
| + | |||
| + | <img src="https://static.igem.org/mediawiki/parts/7/72/Top10woIPTG.JPG" style="width: 350px; height: 205.5;"> | ||
| + | <img src="https://static.igem.org/mediawiki/parts/b/bd/DH5woIPTG.jpeg" style="width: 350px; height: 205.5;"> | ||
| + | <img src="https://static.igem.org/mediawiki/parts/b/b7/BL21wolIPTG.jpeg" style="width: 350px; height: 205.5;"> | ||
| + | |||
| + | |||
| + | </html> | ||
==Team INSA-UPS France 2017 : usage in <i>Vibrio harveyi</i> strain engineered by conjugation== | ==Team INSA-UPS France 2017 : usage in <i>Vibrio harveyi</i> strain engineered by conjugation== | ||
Revision as of 17:41, 31 October 2017
RFP Coding Device
The colonies are clearly red in color under natural light after about 18 hours. Smaller colonies are visibly red under UV. The RFP part does not contain a degradation tag and the RBS is strong.
- LacI sensitive
- CAP sensitive
This part is commonly used, but can fail if the system contains LacI or CAP protein.
(--Meagan 15:39, 23 July 2009 (UTC))
[http://2012.igem.org/Team:TU_Munich Team TU_Munich 2012] improved this part by making it compatible to RFC10 and RFC25 (see: BBa_K801100)
(--VolkerMorath 15:02, 21 October 2012 (UTC))
[http://2013.igem.org/Team:NRP-UEA-Norwich Team NRP-UEA 2013] improved this part by adding a NdeI restriction site before the RFP gene. (see: BBa_K1041000)
(--holusac 20:46, 14 August 2013 (UTC))
[http://2015.igem.org/Team:Warwick Team Warwick 2015] improved this part by analysing the effect of copy number on gene expression.
(--Lcarroll 20:48, 25 September 2015 (UTC))
[http://2016.igem.org/Team:Leiden Team Leiden 2016] contributed to the characterisation of this part by showing equal functionality in simulated microgravity (0g) as in the normal gravity of the Earth.
(--Valentijn 19:38, 19 October 2016 (UTC))
[http://2017.igem.org/Team:UChicago Team UChicago 2017] contributed to this part by improving/changing the documented sequence through mutagenesis to create blunt-end restriction sites for cloning not within the prefix/suffix region (created BBa_K2428000).
(--pzulueta97 21:14, 25 October 2017 (UTC) )
[http://2017.igem.org/Team:Grenoble-Alpes Team Grenoble-Alpes 2017] contributed to the characterisation of this part by testing the time of apparition of fluorescence, in presence of IPTG or not (because the promoter leaks), as well as they contributed to the improvement of this part by using its fluorescence as a detection signal to be able to detect Vibrio Cholerae.
(--NoreenLouis 20:47, 26 October 2017 (UTC) )
[http://2017.igem.org/Team:Kingsborough_NY Team Kingsborough NY 2017] contributed to the characterization of this part by showing decreased fluorescence when expressed either in a higher salt media - such as LB with 3% sodium chloride - or E. coli that lacks tmRNA, the principal component of the cell's ribosome rescue system. [http://2017.igem.org/Team:Kingsborough_NY/RFP See the data at our Wiki]
(--djcamenares 17:56, 27 October 2017 (UTC) )
[http://2017.igem.org/Team:iTesla-SoundBio Team iTesla SoundBio 2017] contributed to the characterization of this part by analyzing the rate of false positives when using the coloring of transformed colonies as a red/white screen for determining experimental success.
(--gladish 01:26, 28 October 2017 (UTC) )
Pictures
Team ITB_Indonesia 2017: Red color dynamics of cloned Escherichia coli strains in LB broth

In Team ITB_Indonesia 2017
characterization, we found in normal growth/incubation condition (37 oC, LB agar) that
BBa_J04450-transformed Escherichia coli BL21 colony appear to need longer incubation time (>18
hours) until it clearly shows red color under natural light.
We then investigate whether this phenomenon is influenced by the strain, and we try if there are
lac repressor in the system that can be released by inducing the culture with IPTG, hence
increasing the expression of mRFP.
Experimental Design
We used three different strains of transformed E. coli (BL21, DH5alpha, and Top10) for this
study. They were incubated in LB broth, 37 oC, and sampled every 4 hours for 2 days to
determine the red color absorbance at 588 nm. The amount of IPTG added for respective treatment is 500
µM.
Result and Findings
- There are no significant differences of mRFP expression in different strains of E. coli (BL21, DH5alpha, Top10)
- There are no significant effects of mRFP increased expression after IPTG induction.
- The red color absorbance under 588 nm wavelength is recorded around 2.5-3 OD units.
- The broth become red in color under natural light around 16-20 hours of incubation time.



Team INSA-UPS France 2017 : usage in Vibrio harveyi strain engineered by conjugation
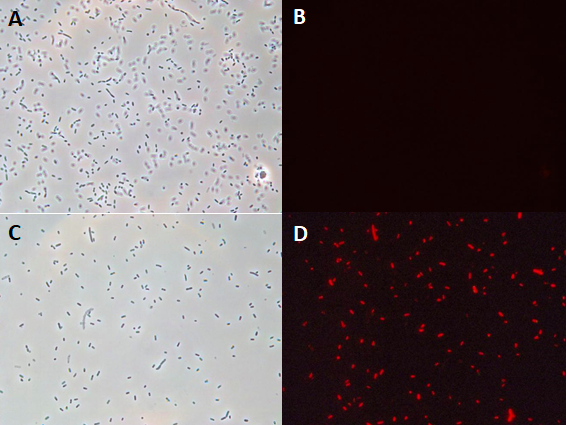
In the context of the iGEM INSA-UPS France project 2017, BBa_J04450 was tested in the Vibrio harveyi background. To the best of our knowledge, RFP has never been used in this strain. BBa_J04450 biobrick was cloned in a broad host range plasmid (pBBR1MCS-4) and conjugated into V. harveyi. The protocol of triparental mating can be found here. Its expression has been studied by fluoresence microscopy in Vibrio harveyi .
The microscopy results demonstrated the fonctional production of RFP in Vibrio harveyi, and hence, the functionality of part BBa_J04450 in this background.
IIT Madras 2016's Characterization
Experimentation
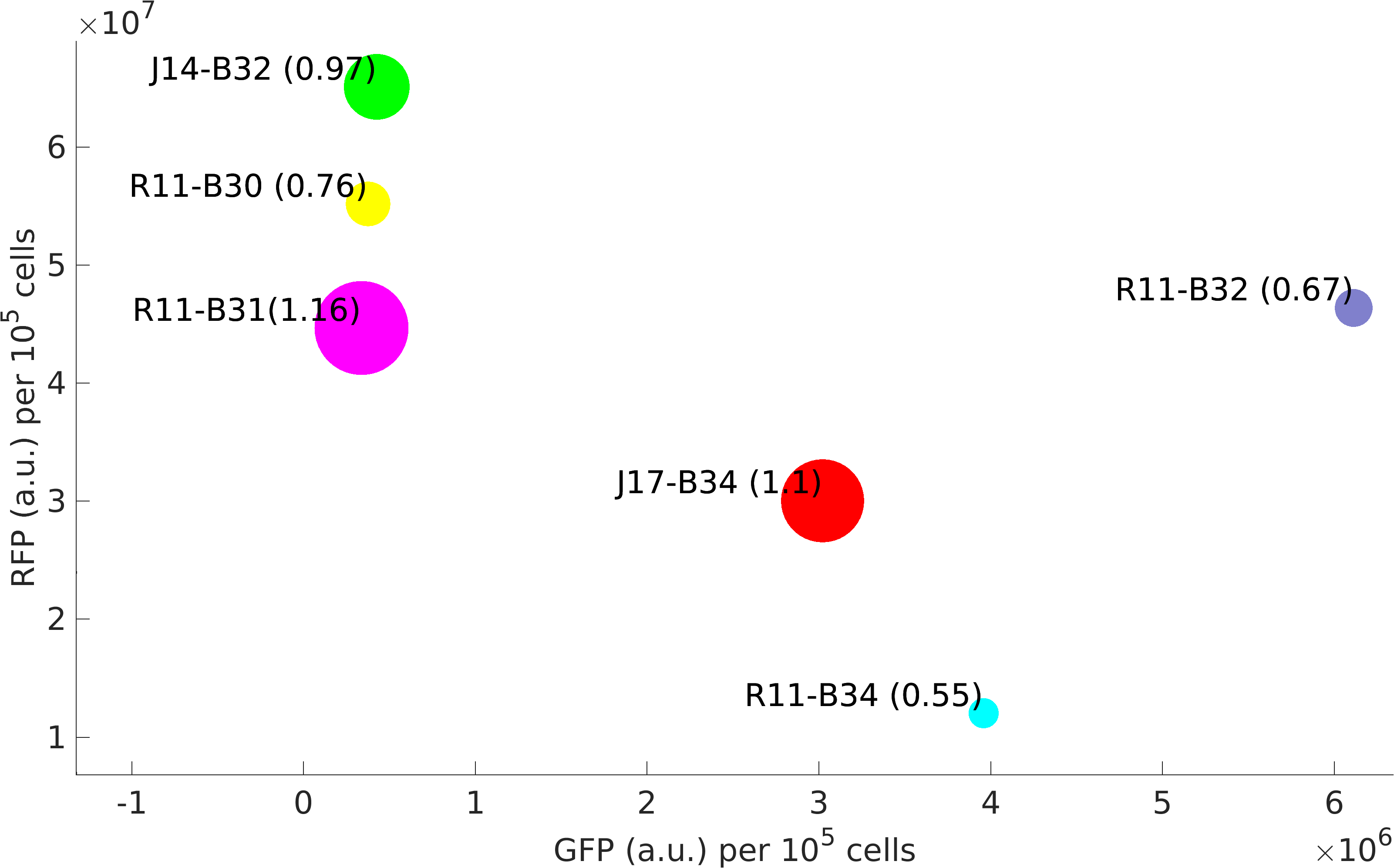
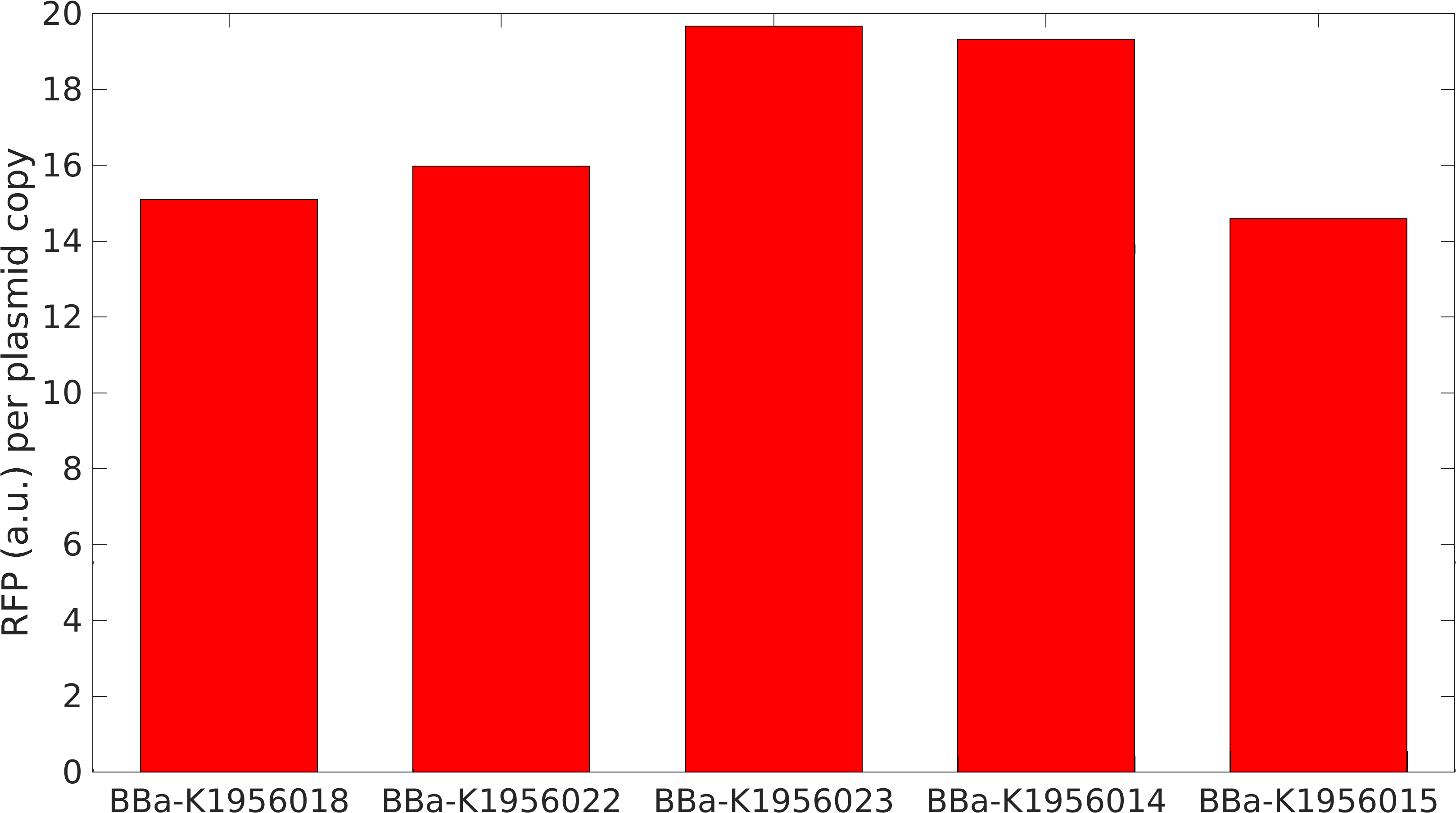
This BioBrick was used along with various GFP producing devices to understand the role of RBS and Promoter parts in giving rise to intrinsic noise in E. coli DH5alpha. Expression data for GFP and RFP proteins were obtained using flow cytometry (BD FACS Aria III) at 3hr, 6hr, 9hr and 12hr stage of growth along with cells expressing only GFP, only RFP and none. Cumulative intrinsic and extrinsic noise were measured using modified [http://2016.igem.org/Team:IIT-Madras/Model#Noise_in_Devices| Elowitz formula]. OD600 values for specific growth rate estimation were obtained using Spectrophotometer over an interval of an hour for 12 hours. Given specific growth rates are in it's logarithmic values. This BioBrick can be used to characterize noise and strength of complex devices by cloning this device with given device, which produces a different reporter protein. In graphs, we have R11-B32, R11-B34, J14-B3, J17-B34, R11-B30 and R11-B31 in pSB1A2 plasmid backbone.
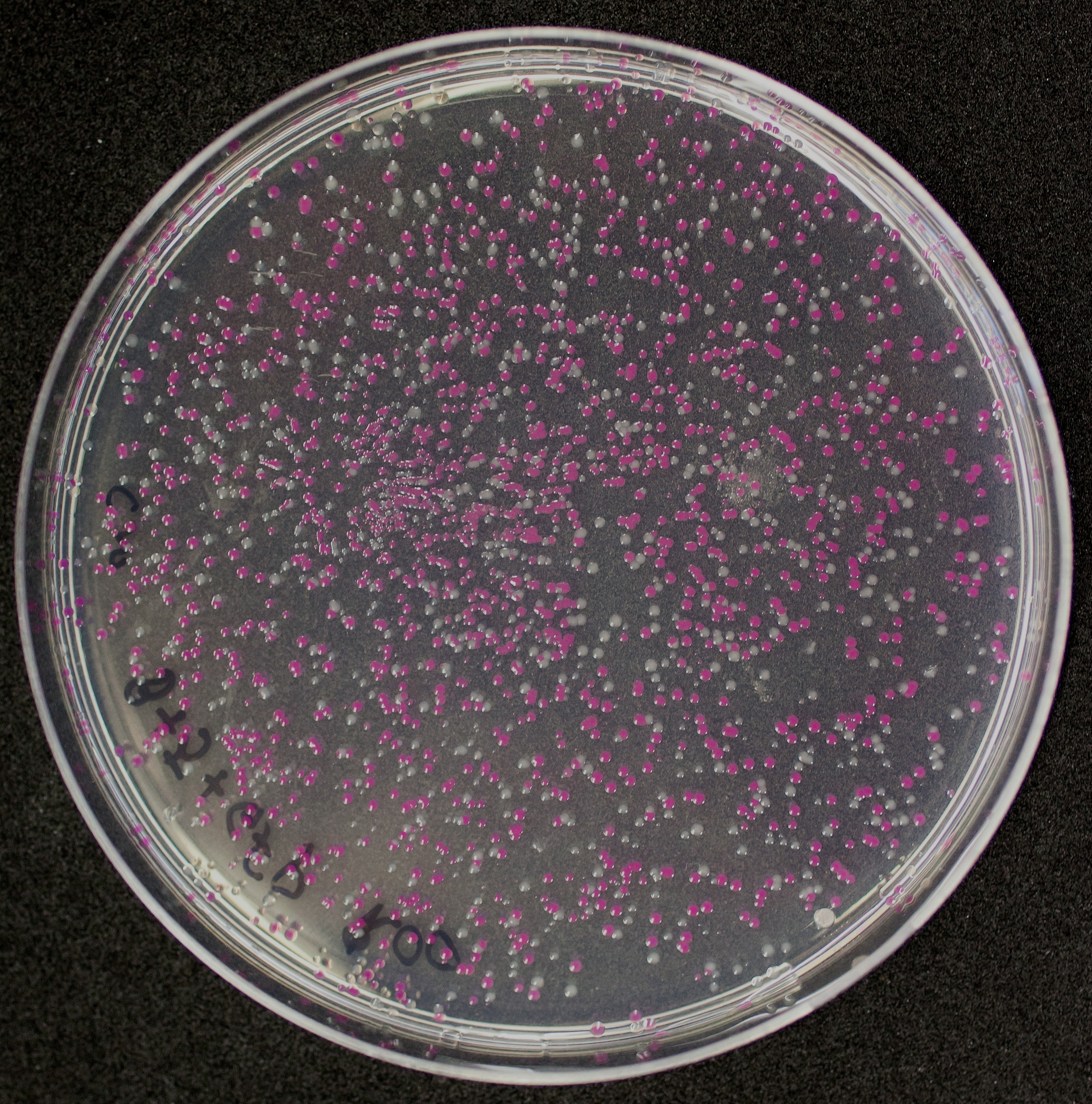
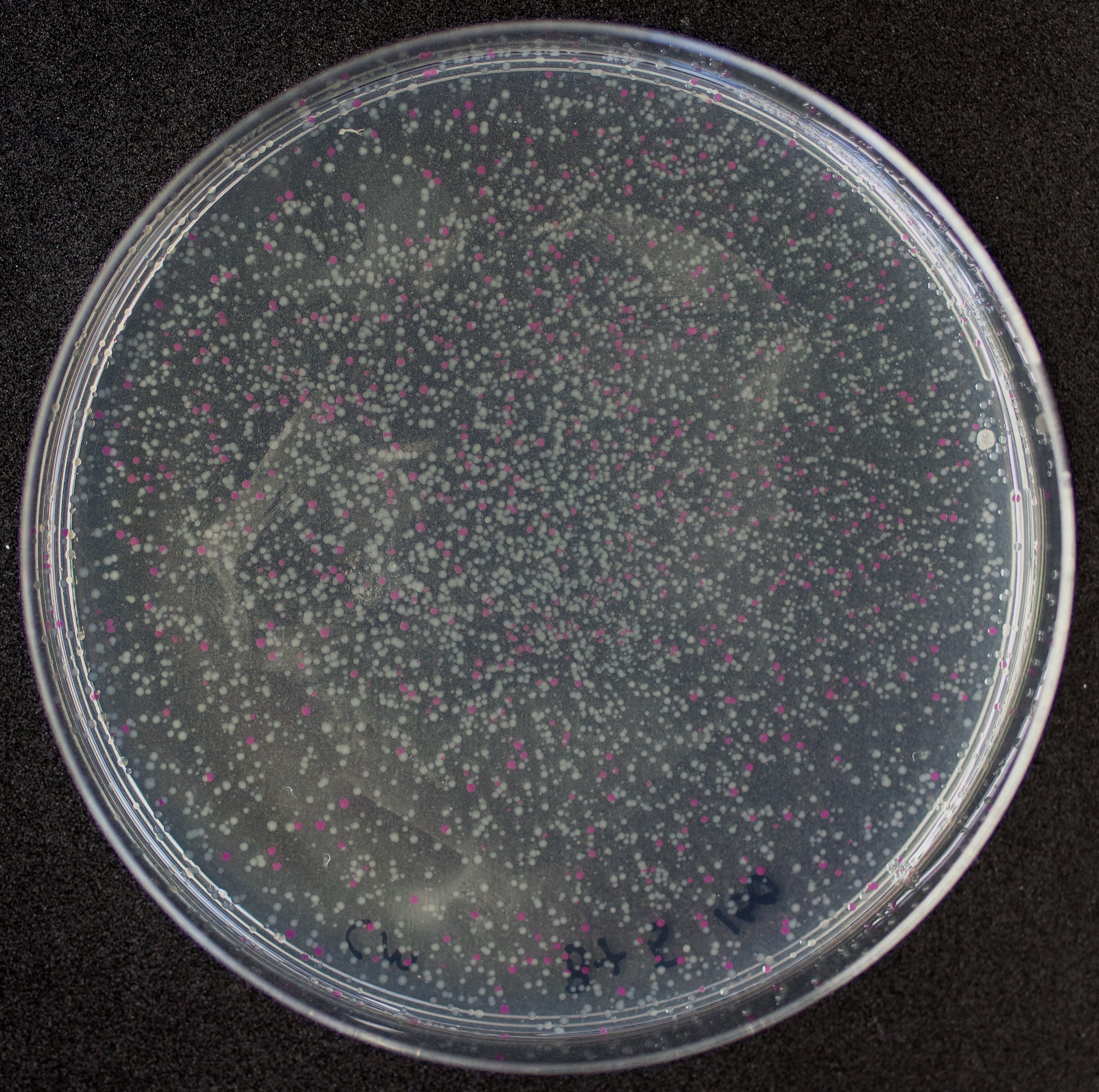
[http://2010.igem.org/Team:Groningen Team Groningen 2010] reports the usage of this part as a cloning tool. When ligating any part, or part assembly, into any standard backbone that contains this part, the non-restricted and single-restricted backbones that self-circularize will produce red colonies on rich media plates (we use TY). These undesired transformants can than be avoided in the screening for the correct construct. With this method, the backbone desired for a new construct does not need to be purified from agarose gel to decrease the amount of undesired tranformants caused by ligation of the original part present in the backbone. The amount of incorrect transformants depends, of course, on the ratio of backbone (mixed with J04450) vs. BioBrick insert, the size of the BioBrick insert, and whether the insert is an assembly of two BioBricks. The images below show two ligations with different efficiencies.
Usage in Chromobacterium Violaceum
[http://2016.igem.org/Team:Tec-Monterrey Team Tec-Monterrey 2016] characterized the output of the part BBa_J04450 in a novel chassis, Chromobacterium Violaceum, as it produces a native purple pigment Violacein, we were curious whether RFP would be useful as a reporter gene. Furthermore, we characterized its expression under lac promoter. We did the transformation of C. Violaceum by a method that has not been reported yet, we made C. Violaceum competent cells with the protocol that is in our wiki, we concluded that the best O.D. for the heat shock transformation is 0.5 since it showed clearly better results than 0.4 or 0.6, we will continue to work in the transformation efficiency.
[Image: ]
]