Difference between revisions of "Part:BBa K103006"
Weihongyang (Talk | contribs) |
(→results) |
||
| Line 17: | Line 17: | ||
===results=== | ===results=== | ||
| − | |||
| − | |||
| − | |||
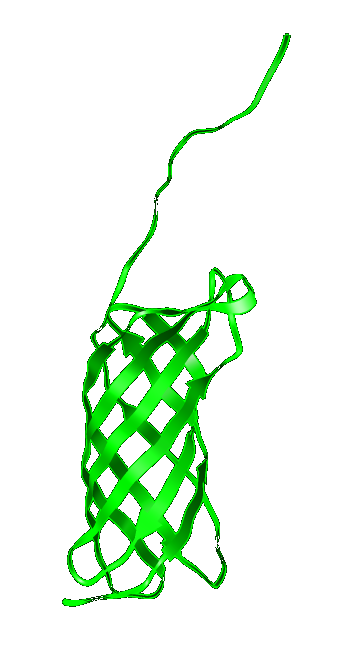
| [[Image:Ompalinker.jpg|200px]] | | [[Image:Ompalinker.jpg|200px]] | ||
This protein structure was predicted using threading and protein fold prediction and may differ from actual one. | This protein structure was predicted using threading and protein fold prediction and may differ from actual one. | ||
|} | |} | ||
| + | E.coli was induced in 37℃ and membrane protein was separated by a speeding centrifuge. The result of western blot showed the difference at the expressed levels of Lpp-ompA-L-FABP in E.coli transformed by our recombinant plasmid and plasmid in registry of iGEM. | ||
| + | https://static.igem.org/mediawiki/parts/2/22/QQ%E5%9B%BE%E7%89%8720171027142246.jpg | ||
<!-- --> | <!-- --> | ||
Revision as of 09:04, 27 October 2017
OmpA outer membrane protein A fused to linker; displays proteins on cell surface
One of our basic bricks used to create fusions attached to outer membrane
E.coli was induced in 37℃ and membrane protein was separated by a speeding centrifuge. The result of western blot showed the difference at the expressed levels of Lpp-ompA-L-FABP in E.coli transformed by our recombinant plasmid and plasmid in registry of iGEM.

Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]