Difference between revisions of "Part:BBa K2230012"
| Line 19: | Line 19: | ||
===Functional assay for glucose responsive suppressor system=== | ===Functional assay for glucose responsive suppressor system=== | ||
| − | [[File:Mingdaophil1025-10.jpeg| | + | [[File:Mingdaophil1025-10.jpeg|600px]] |
The bacteria carrying the indicated vector were cultured in LB media supplemented with 34 g/ml of chloramphenicol (Cm) at 37°C overnight. The next day, OD600 was measured and adjusted to 2.5 in M9 minimal media with various concentrations of glucose. The bacteria then were incubated for 4 hours at 37°C. 100 l of the bacterial culture was put into one well of a black-walled, clear-bottom 96-well microplates (Thermo Fisher Scientific Inc.). The fluorescence intensity was measured using BioTek Synergy H1 Hybrid Multi-Mode Reader System at Ex/Em = 488nm/518nm for GFP. | The bacteria carrying the indicated vector were cultured in LB media supplemented with 34 g/ml of chloramphenicol (Cm) at 37°C overnight. The next day, OD600 was measured and adjusted to 2.5 in M9 minimal media with various concentrations of glucose. The bacteria then were incubated for 4 hours at 37°C. 100 l of the bacterial culture was put into one well of a black-walled, clear-bottom 96-well microplates (Thermo Fisher Scientific Inc.). The fluorescence intensity was measured using BioTek Synergy H1 Hybrid Multi-Mode Reader System at Ex/Em = 488nm/518nm for GFP. | ||
| − | [[File:Mingdaophil1025-11.png| | + | [[File:Mingdaophil1025-11.png|500px|center]] |
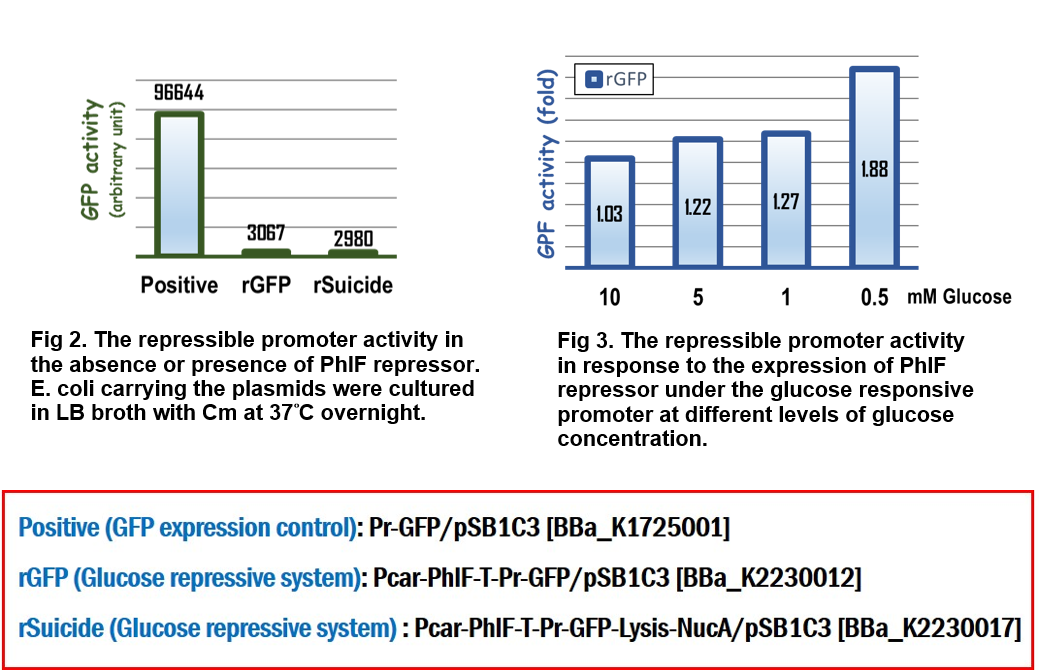
In the assay for repressor system, the data in Fig. 2 gave the similar results as team [http://2015.igem.org/Team:Glasgow/Project/Overview/Repressors Glasgow] did in 2015, in which the strong activity of the repressible promoter was significantly repressed in the presence of PhlF repressor. | In the assay for repressor system, the data in Fig. 2 gave the similar results as team [http://2015.igem.org/Team:Glasgow/Project/Overview/Repressors Glasgow] did in 2015, in which the strong activity of the repressible promoter was significantly repressed in the presence of PhlF repressor. | ||
| Line 29: | Line 29: | ||
In the assay for glucose responsive repressor system, we improved the Glasgow’s BioBrick device, in which the expression of PhlF repressor was driven by a glucose response promoter, Pcar. The result in Fig 3 clearly indicated that the GFP activity driven by the repressible promoter was gradually increased in response to the loss of glucose to 1.88 folds compared to the initial GFP activity at the beginning culture in M9 media, suggesting that the level of expression of PhlF was positively corresponding to the concentration of glucose. | In the assay for glucose responsive repressor system, we improved the Glasgow’s BioBrick device, in which the expression of PhlF repressor was driven by a glucose response promoter, Pcar. The result in Fig 3 clearly indicated that the GFP activity driven by the repressible promoter was gradually increased in response to the loss of glucose to 1.88 folds compared to the initial GFP activity at the beginning culture in M9 media, suggesting that the level of expression of PhlF was positively corresponding to the concentration of glucose. | ||
| − | [[File:Mingdaophil1025-12.png| | + | [[File:Mingdaophil1025-12.png|400p|center]] |
Revision as of 08:28, 25 October 2017
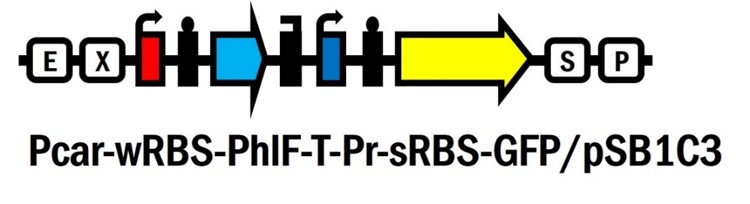
Pcar-wRBS-PhlF-T-Pr-sRBS-GFP/pSB1C3
Promoter Pcar [BBa_K861171] is a glucose responsive promoter created by WHU-China in 2012. Pcar promoter region was de novo designed with overlapping of CRP and RNA polymerase binding site. The initiation of transcription by RNA polymerase may be hindered by the binding of CRP, which occurs at the formation of cAMP-CRP complex in the low concentration of glucose. In other words, when the amount of glucose is high enough, Pcar would be turned on after the leaving of CPR due to the low concentration of cAMP, and vice versa.
PhlF repressor system contains the repressor PhlF [BBa_K1725041] and the PhlF repressible promoter [BBa_K1725001] created by Glasgow in 2015. PhlF could repress GFP fluorescence intensity by 83-fold according to the study of Glasgow’s work.
We’ve innovated this year a novel glucose responsive repressor system (Pcar-wRBS-PhlF-T-Pr-sRBS-GFP/pSB1C3 [BBa_K2230012]) by connecting these two system and extend the function of them. Furthermore, based on this new system, we assembled lysis and nuclease genes to the device and created the suicide circuit controlled by the presence of glucose (Pcar-wRBS-PhlF-T-Pr-sRBS-GFP-sRBS-lysis-sRBS-NucA/pSB1C3 [BBa_K2230017]).
Cloning
Pr-sRBS-GFP (BBa_K1725001) with strong RBS (B0034) was assembled with Pcar-RBS-PhlF-T/pSB1C3 (BBa_K2230010)
Function
The expression of GFP driven by PhlF-repressed promoter (named Pr here) was dose-dependently regulated by PhlF repressor and reduced upon an increasing concentrations of glucose. In other words, the intensity of GFP increased when glucose gradually ran out.
Functional assay for glucose responsive suppressor system
The bacteria carrying the indicated vector were cultured in LB media supplemented with 34 g/ml of chloramphenicol (Cm) at 37°C overnight. The next day, OD600 was measured and adjusted to 2.5 in M9 minimal media with various concentrations of glucose. The bacteria then were incubated for 4 hours at 37°C. 100 l of the bacterial culture was put into one well of a black-walled, clear-bottom 96-well microplates (Thermo Fisher Scientific Inc.). The fluorescence intensity was measured using BioTek Synergy H1 Hybrid Multi-Mode Reader System at Ex/Em = 488nm/518nm for GFP.
In the assay for repressor system, the data in Fig. 2 gave the similar results as team [http://2015.igem.org/Team:Glasgow/Project/Overview/Repressors Glasgow] did in 2015, in which the strong activity of the repressible promoter was significantly repressed in the presence of PhlF repressor.
In the assay for glucose responsive repressor system, we improved the Glasgow’s BioBrick device, in which the expression of PhlF repressor was driven by a glucose response promoter, Pcar. The result in Fig 3 clearly indicated that the GFP activity driven by the repressible promoter was gradually increased in response to the loss of glucose to 1.88 folds compared to the initial GFP activity at the beginning culture in M9 media, suggesting that the level of expression of PhlF was positively corresponding to the concentration of glucose.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1501