Difference between revisions of "Part:BBa C0071"
| Line 73: | Line 73: | ||
If you want more information, you see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]! | If you want more information, you see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]! | ||
| + | |||
| + | Group: <b>Imperial College London 2016</b> | ||
| + | |||
| + | ====I. Characterisation of RhlR by Imperial College London iGEM 2016 ==== | ||
| + | |||
| + | We improved this part by characterising the crosstalk between RhlR and non-cognate AHLs. We found the activation range of RhlR when induced with C4-AHL. For the crosstalk experiments, we tested C12-AHL, C14-AHL, and C6-AHL. We placed the coding sequence downstream from the pRhl promoter, and recorded the fluorescence for different concentrations of AHLs. | ||
| + | |||
| + | We found that there was no significant activation of RhlR by the C12-AHL. | ||
| + | |||
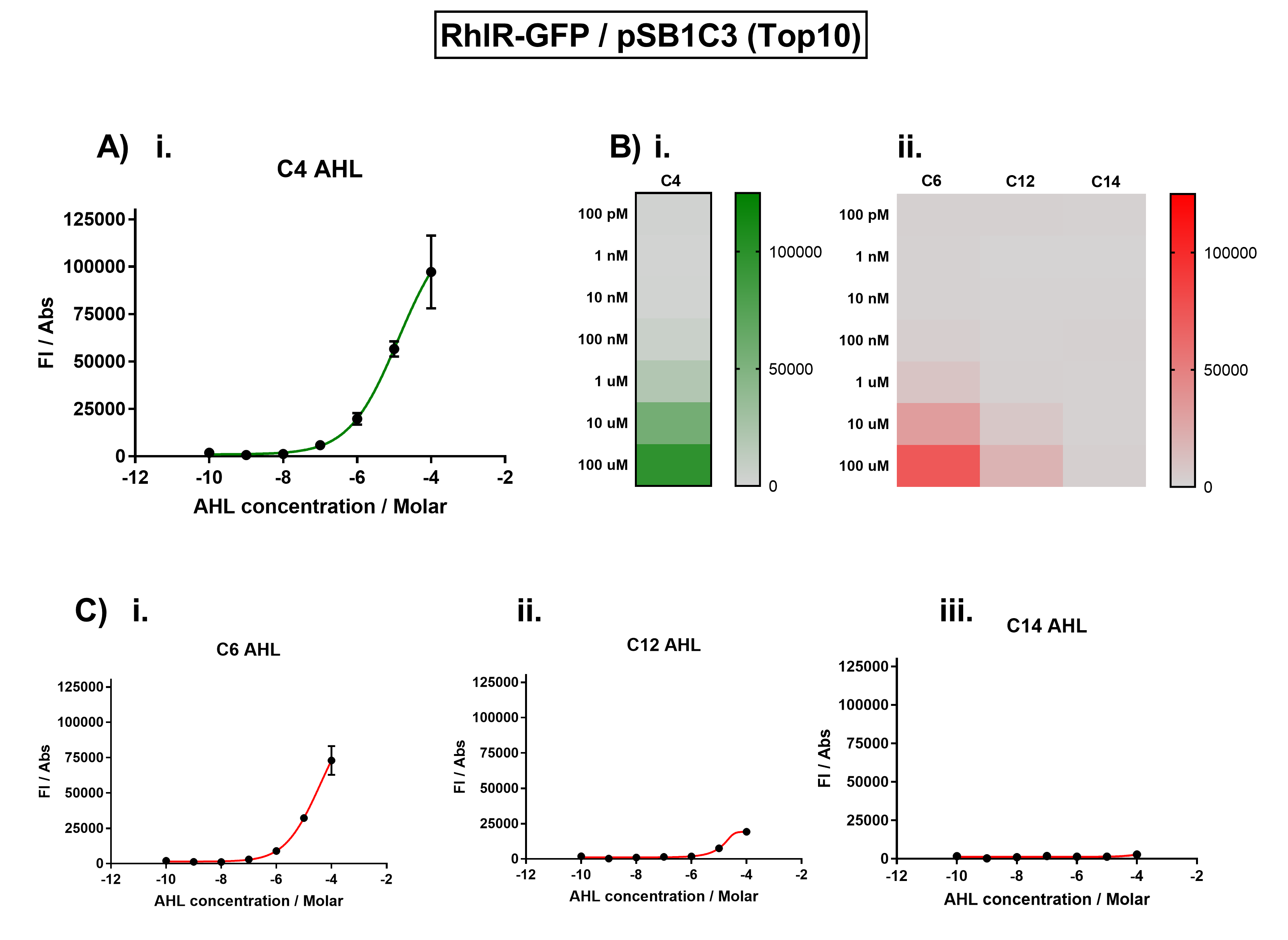
| + | [[File:IC16_BBa_K1893003_characterisation.png|700px|center|]] | ||
| + | Figure 1. Characterisation of the Rhl response device (BBa_K1893003). (A) Transfer function curve of normalised fluorescence against cognate inducer C4-AHL concentrations. (B) Heat map of normalised fluorescence of RhlR-GFP system over a range of AHL concentrations: (i) Binding of RhlR-GFP to its cognate AHL (C4 AHL). (ii) Binding of RhlR-GFP to 3 non-cognate AHLs (3O-C6 AHL, 3O-C12 AHL, 3OH-C14 AHL). (C) Transfer function curves of normalised fluorescence against non-cognate inducer AHL (C4 AHL) concentrations to investigate inducer AHL crosstalk: (i) C6-AHL (3O-C6 AHL) of the Lux system (ii) C12-AHL (3O-C12 AHL) of the Las system (iii) C14-AHL (3O-C14 AHL) of the Cin system. Experiments were performed in E. coli Top10 cell strain cultured at 37°C. Normalised fluorescence was calculated by dividing fluorescent signal by cell density (OD600). Fluorescence measurements were recorded at 180 minutes. Reported values represent the mean normalised fluorescence value from 3 technical repeats and error bars represent standard deviation of these. | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
Revision as of 13:48, 23 October 2016
rhlR repressor/activator from P. aeruginosa PA3477 (+LVA)
Transcriptional regulator, in complex with N-butyryl-HSL, RhlR binds to the Rhl promoter
Usage and Biology
Transcriptional regulator, binds with N-butyryl-HSL
Characterization
Group: Tokyo Tech 2016
Author: Yoshio Takata
Summary of Improvement and Characterization:
I. Improved Prhl by iGEM 2014 Tokyo_Tech team and characterize RhlR assay
II. Improvement of the wild type Prhl
III. Comparison of the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl
We Tokyo Tech 2016 simulated our final genetic circuits and found that the circuits did not work, because Prhl strength was too weak. (see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]). We therefore considered using the improved Prhl (BBa_K1529310,BBa_K1529310) established by Tokyo_Tech 2014, but we noticed that they were inappropriate for two reasons (see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]). Then, we decided to improve Prhl Promoter and obtain our original improved Prhl (included in BBa_K1949060) that suited our goal.
Our purpose is to create strong Prhl for our final genetic circuits. This experiment consists of the three parts below.
I. Improved Prhl by iGEM 2014 Tokyo_Tech team and characterize rhlR assay
II. Improvement of the native Prhl
III. Comparison of the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl
The past improved Prhl did not suit for our final circuits and we could create the improved Prhl appropriate to our final circuits.
I. Improved Prhl by iGEM 2014 Tokyo_Tech item and characterize RhlR assay
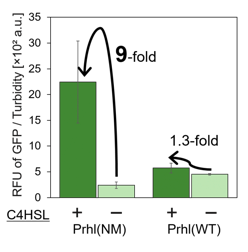
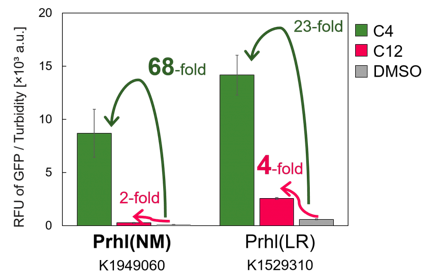
We found that Prhl(RL) (BBa_K1529300) activity was weak and the expression level depended on LVA tag (Fig.1); LVA-tagged proteins are prone to be degraded by cellular proteases. Prhl(LR) (BBa_K1529310) activity was strong and unexpectedly reacted with C12 (crosstalk) (Fig.3).
The colonies of transformants with a rhlR (BBa_C0171) plasmid looked rough and the growth rate was low(Fig.2-left), while the colonies of transformants with a rhlR-LVA (BBa_C0071) plasmid looked smooth and the growth rate was normal(Fig.2-right). However, the reason for this result is unclear.
II. Improvement the wild type Prhl
By introducing a single point mutation into wild type Prhl (BBa_R0071) by PCR, we obtained 198 Prhl mutants and chose the two Prhl mutants of which promoter activity was stronger than wild type Prhl(Fig.3).
The sequences of these two chosen mutants are shown below. The small characters indicate the scar sequence and a single point mutation is colored with red.
Native Prhl (BBa_R0071)
TCCTGTGAAATCTGGCAGTTACCGTTAGCTTTCGAATTGGCTAAAAAGTGTTC
Prhl(NM)(BBa_K1949060)
TCCTGTGAAATCTGGCAGTTACCGTTAGCTTTCGAATTGGCTAtAAAGTGTTC
III. Comparison the improved Prhl by iGEM 2014 Tokyo_Tech team to our original improved Prhl
Prhl(NM) was chosen from the many Prhl mutants, and by comparing Prhl(NM) to Prhl(LR), we obtained the result below (Fig.4). The reaction activity of Prhl(NM) to C4 was stronger than that of Prhl(LR), and Prhl(NM) did not react with C12 at all.
If you want more information, you see [http://2016.igem.org/Team:Tokyo_Tech our work in Tokyo_Tech 2016 wiki]!
Group: Imperial College London 2016
I. Characterisation of RhlR by Imperial College London iGEM 2016
We improved this part by characterising the crosstalk between RhlR and non-cognate AHLs. We found the activation range of RhlR when induced with C4-AHL. For the crosstalk experiments, we tested C12-AHL, C14-AHL, and C6-AHL. We placed the coding sequence downstream from the pRhl promoter, and recorded the fluorescence for different concentrations of AHLs.
We found that there was no significant activation of RhlR by the C12-AHL.
Figure 1. Characterisation of the Rhl response device (BBa_K1893003). (A) Transfer function curve of normalised fluorescence against cognate inducer C4-AHL concentrations. (B) Heat map of normalised fluorescence of RhlR-GFP system over a range of AHL concentrations: (i) Binding of RhlR-GFP to its cognate AHL (C4 AHL). (ii) Binding of RhlR-GFP to 3 non-cognate AHLs (3O-C6 AHL, 3O-C12 AHL, 3OH-C14 AHL). (C) Transfer function curves of normalised fluorescence against non-cognate inducer AHL (C4 AHL) concentrations to investigate inducer AHL crosstalk: (i) C6-AHL (3O-C6 AHL) of the Lux system (ii) C12-AHL (3O-C12 AHL) of the Las system (iii) C14-AHL (3O-C14 AHL) of the Cin system. Experiments were performed in E. coli Top10 cell strain cultured at 37°C. Normalised fluorescence was calculated by dividing fluorescent signal by cell density (OD600). Fluorescence measurements were recorded at 180 minutes. Reported values represent the mean normalised fluorescence value from 3 technical repeats and error bars represent standard deviation of these.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 240
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 715