Difference between revisions of "Part:BBa K1887003"
(→Usage and Biology) |
|||
| Line 14: | Line 14: | ||
| − | === | + | ===Molecular verification=== |
| + | We used PCR and restriction enzyme digestion to verify the cA part on pSB1C3. | ||
| + | |||
| + | [[file:cAm.jpg]] | ||
| + | |||
===Background=== | ===Background=== | ||
To build a surface display system, the first thing should be considered is the cell wall anchoring motif. The most exploited anchoring regions are those with the LPXTG motifs that bind the proteins in a covalent way to the cell wall. However, to achieve versatile application, it is better to use anchoring domains that interact with the cell wall in a non-covalent way. The major autolysin of ''L. lactis'', the cell wall hydrolase AcmA contains 3 tandem arranged LysM motifs and separated by stretches of 21 to 31 amino acids, this region is collectively termed as the cA anchoring domain. The cA domain can be fused to the N- and C- terminus of functional proteins, and can bind proteins to the cell walls of a broad range of gram-positive bacteria. | To build a surface display system, the first thing should be considered is the cell wall anchoring motif. The most exploited anchoring regions are those with the LPXTG motifs that bind the proteins in a covalent way to the cell wall. However, to achieve versatile application, it is better to use anchoring domains that interact with the cell wall in a non-covalent way. The major autolysin of ''L. lactis'', the cell wall hydrolase AcmA contains 3 tandem arranged LysM motifs and separated by stretches of 21 to 31 amino acids, this region is collectively termed as the cA anchoring domain. The cA domain can be fused to the N- and C- terminus of functional proteins, and can bind proteins to the cell walls of a broad range of gram-positive bacteria. | ||
Revision as of 12:51, 15 October 2016
cA anchoring domain
A cell wall binding domain used in surface display for L. lactis
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Molecular verification
We used PCR and restriction enzyme digestion to verify the cA part on pSB1C3.
Background
To build a surface display system, the first thing should be considered is the cell wall anchoring motif. The most exploited anchoring regions are those with the LPXTG motifs that bind the proteins in a covalent way to the cell wall. However, to achieve versatile application, it is better to use anchoring domains that interact with the cell wall in a non-covalent way. The major autolysin of L. lactis, the cell wall hydrolase AcmA contains 3 tandem arranged LysM motifs and separated by stretches of 21 to 31 amino acids, this region is collectively termed as the cA anchoring domain. The cA domain can be fused to the N- and C- terminus of functional proteins, and can bind proteins to the cell walls of a broad range of gram-positive bacteria.
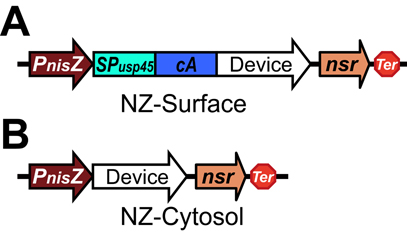
Design
The protein of interest is fused to the cA domain with the USP45 signal peptide, driven by the PnisZ promoter and followed by the nisin resistant gene nsr.
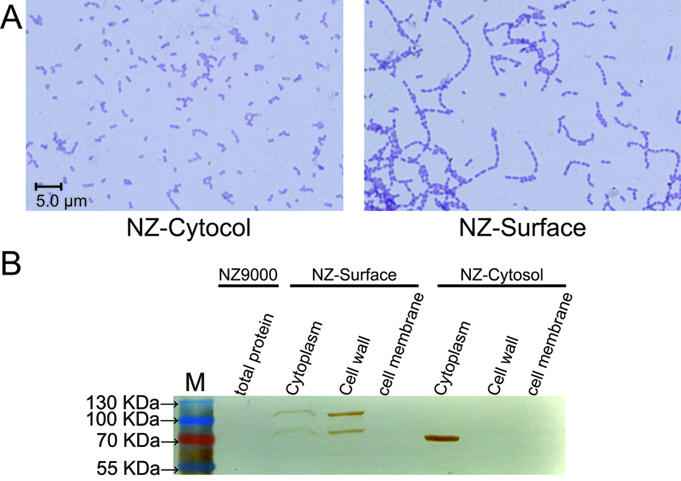
Results
To demonstrate whether cA could indeed anchor the protein of interest at the surface of L. lactis, the pLacZ-Cytosol and pLacZ-Surface plasmids were introduced into NZ9000 respectively. As stated above, the cA domain is derived from the AcmA protein, which is an autolysin of L. latis that cleaves the peptidoglycan to release the duplicated bacteria. Since the substrate peptidoglycan is now occupied by the cA-β-galactosidase fusion proteins, the AcmA autolysin activity is hindered, thus cell separation will be interfered. Indeed, we found that under microscopic, the NZ-Surface cells were poorly separated compared to the NZ-Cytosol strain. Further more, using a polyclonal antibody against β-galactosidase, we found that about 70% of the β-galactosidase protein is present at the cell wall, while the other 30% protein is present in the cytoplasm, perhaps due to the inefficient secretion process. In contrast, all the β-galactosidase proteins were present in the cytoplasm in the NZ-cytosol strain.
References
[1] Buist, G., Steen, A., Kok, J., and Kuipers, O.P. (2008). LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol 68, 838-847.
[2] Raha, A.R., Varma, N.R., Yusoff, K., Ross, E., and Foo, H.L. (2005). Cell surface display system for Lactococcus lactis: a novel development for oral vaccine. Appl Microbiol Biotechnol 68, 75-81.