Difference between revisions of "Part:BBa I715019"
(→Functional Test) |
Qiuxinyuan12 (Talk | contribs) |
||
| Line 16: | Line 16: | ||
<partinfo>BBa_I715019 parameters</partinfo> | <partinfo>BBa_I715019 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | |||
| + | |||
| + | ==Contribution: NUDT_CHINA 2016== | ||
| + | Author: Xinyuan Qiu | ||
| + | |||
| + | Summary: In this contribution, we further improved the characterization of BBa_ I715019 by optimizing its expression parameters. Also, we introduced another split-GFP system based on the sequence provided by BBa_ I715019 and BBa_ I715020 with a better noise-signal ratio compared to the traditional approaches. | ||
| + | |||
| + | <html> | ||
| + | <p style="text-indent:22pt;"> | ||
| + | <span style="line-height:1;font-family:Perpetua;font-size:16px;">To | ||
| + | further improve the function of existing parts, we stimulate an </span><i><span style="line-height:1;font-family:Perpetua;font-size:16px;">in vivo</span></i><span style="line-height:1;font-family:Perpetua;font-size:16px;"> PPI situation, and tried to | ||
| + | optimize the culture condition for a better signal-to-noise ratio (SNR). For | ||
| + | such matter, two devices, containing split-GFP fragments and a complete or | ||
| + | spited zinc finger protein, were built under control of a lac operon controlled | ||
| + | T7 promoter. The complete zinc finger protein was to stimulate a PPI positive | ||
| + | situation, while the split one was to stimulate a PPI negative situation | ||
| + | . Fluorescence signal was detected by a microplate reader after an | ||
| + | overnight culture under various conditions.</span><span style="line-height:1;font-family:Perpetua;font-size:16px;"> </span><span style="line-height:1;font-family:Perpetua;font-size:16px;">Relative fluorescence intensity was then calculated with normalization | ||
| + | of OD</span><sub><span style="line-height:1;font-family:Perpetua;font-size:16px;">600</span></sub><span style="line-height:1;font-family:Perpetua;font-size:16px;"> value. The relative fluorescence intensity of each control | ||
| + | group was set arbitrarily at 1.0 (data not shown), and the levels of the other | ||
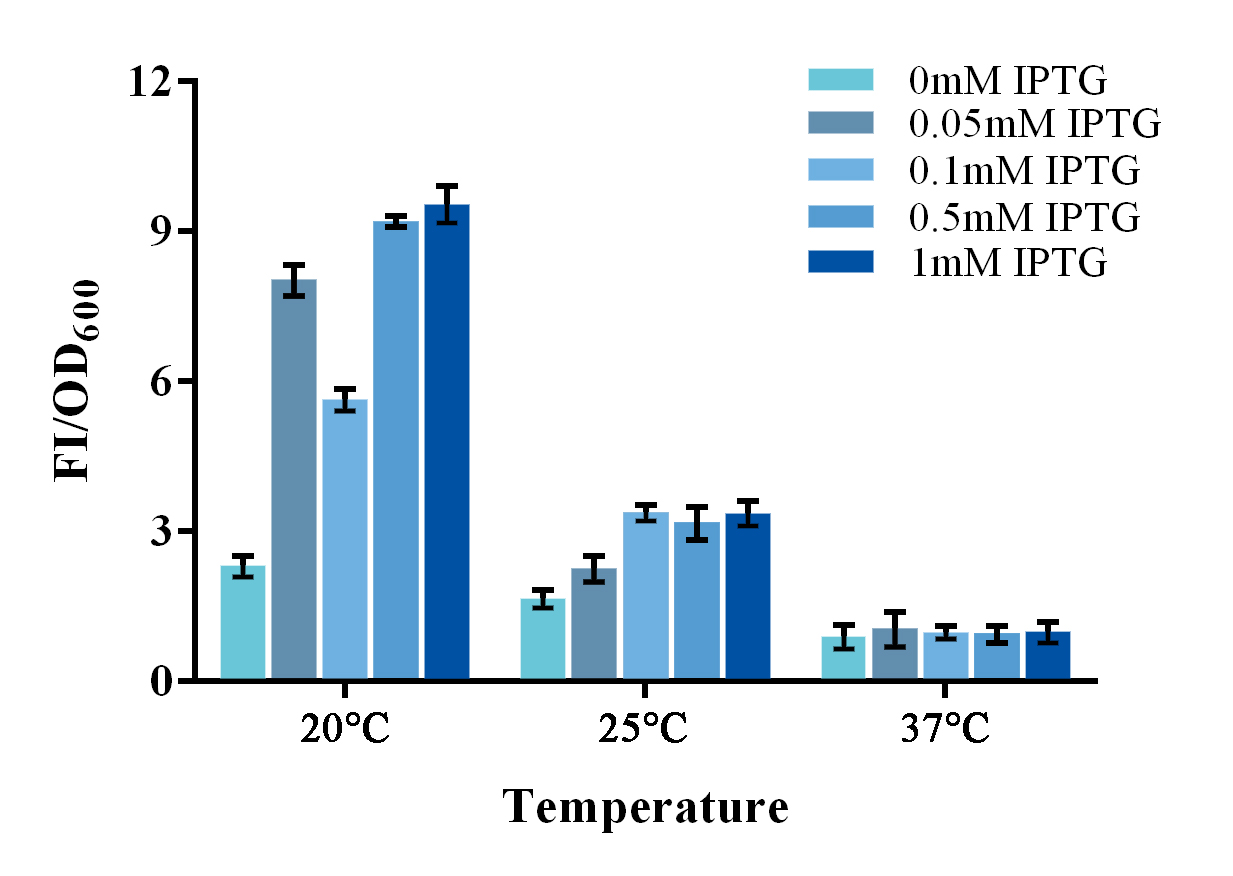
| + | groups were adjusted correspondingly. Results shown a better SNR under 20</span><span style="line-height:1;font-family:Perpetua;font-size:16px;">℃</span><span style="line-height:1;font-family:Perpetua;font-size:16px;"> and 0.5mM IPTG induction (Figure 3). Thus indicating that better performance | ||
| + | of such system could be expected under lower culturing temperature.</span> | ||
| + | </p> | ||
| + | <p style="text-indent:22pt;"> | ||
| + | <span style="line-height:1;font-family:Perpetua;font-size:16px;"> </span> | ||
| + | </p> | ||
| + | |||
| + | </br> | ||
| + | </html> | ||
| + | [[File:T--NUDT CHINA--introfig3.jpg|700px|center]] | ||
| + | <html> | ||
| + | </br> | ||
| + | <p> | ||
| + | <b><span style="line-height:1;font-family:Perpetua;font-size:16px;">Figure 3. Evaluation of the split-GFP system under different expression condition. </span></b> | ||
| + | </p> | ||
| + | <p> | ||
| + | <span style="line-height:1;font-family:Perpetua;font-size:16px;">Relative fluorescence intensity was calculated with normalization of the OD600 value. The relative fluorescence intensity of each control group was set arbitrarily at 1.0 (data not shown), and the levels of the other groups were adjusted correspondingly. Green fluorescence was measured under 488nm of excitation and 538nm of emission. This experiment was run in three parallel reactions, and the data represent results obtained from at least three independent experiments. *p<0.05, **p<0.01.</span> | ||
| + | </p> | ||
| + | </br> | ||
| + | |||
| + | </html> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
Revision as of 17:47, 19 October 2016
Amino Half of GFP (aka GFP1)
A
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Contribution: NUDT_CHINA 2016
Author: Xinyuan Qiu
Summary: In this contribution, we further improved the characterization of BBa_ I715019 by optimizing its expression parameters. Also, we introduced another split-GFP system based on the sequence provided by BBa_ I715019 and BBa_ I715020 with a better noise-signal ratio compared to the traditional approaches.
To further improve the function of existing parts, we stimulate an in vivo PPI situation, and tried to optimize the culture condition for a better signal-to-noise ratio (SNR). For such matter, two devices, containing split-GFP fragments and a complete or spited zinc finger protein, were built under control of a lac operon controlled T7 promoter. The complete zinc finger protein was to stimulate a PPI positive situation, while the split one was to stimulate a PPI negative situation . Fluorescence signal was detected by a microplate reader after an overnight culture under various conditions. Relative fluorescence intensity was then calculated with normalization of OD600 value. The relative fluorescence intensity of each control group was set arbitrarily at 1.0 (data not shown), and the levels of the other groups were adjusted correspondingly. Results shown a better SNR under 20℃ and 0.5mM IPTG induction (Figure 3). Thus indicating that better performance of such system could be expected under lower culturing temperature.
Figure 3. Evaluation of the split-GFP system under different expression condition.
Relative fluorescence intensity was calculated with normalization of the OD600 value. The relative fluorescence intensity of each control group was set arbitrarily at 1.0 (data not shown), and the levels of the other groups were adjusted correspondingly. Green fluorescence was measured under 488nm of excitation and 538nm of emission. This experiment was run in three parallel reactions, and the data represent results obtained from at least three independent experiments. *p<0.05, **p<0.01.
Contribution: NUDT_CHINA 2015
Author: Xinyuan Qiu
Summary: In this contribution, we designed a pair of primers that can extent the usage of this part, and tested its function.
A pair of primers that can extent the usage of this part
In our project, we plan to fuse the N-terminal of GFP (A.K.A. GFP1) to the C-terminal of TALE1 protein (you may visit our wiki for more details). However, the sample of BBa_ I715019 provided by the 2015 DNA distribution does not have a termination codon on its 3’ terminal to stop the translation. To fix this, we designed a pair of primers to add a termination codon on the 3’ terminal of BBa_ I715019 to further extend its usage.
F-Prime: 5’- GAATTCGCGGCCGCTTCTAGAATGC-3’
R-Prime: 5’- GGACTAGTATTATTGTTTGTCTGCC-3’
Functional Test
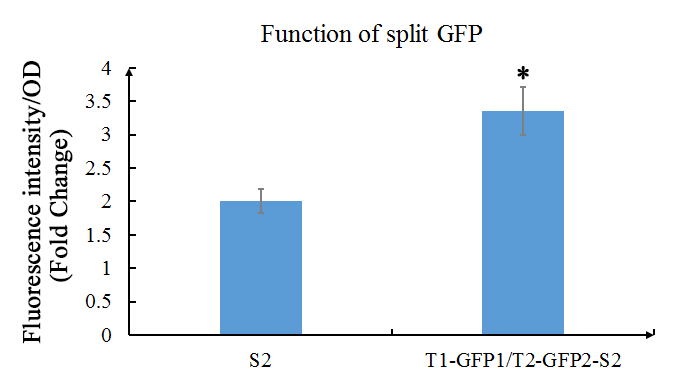
We also used the improved version (using the primers above) of GFP1 and GFP2 (the C- terminal of GFP, that part is also improved) and tested the their functions. In our experiment, GFP1 was fused with TALE1, GFP2 was fused with TALE2; and SCAF2 was added into the plasmid. The plasmid was transformed into E.coli BL21(DE3) and cultured in LB with 30mg/ml Chloramphenicol to OD600=0.6, then inducted with 1mM IPTG overnight.
Fig. 1 Evaluation of the functions of split GFP. The green fluorescence (Ex: 488 nm; Em: 538 nm) of split GFP was detected after overnight culture of E.coli with or without GFP1/2 under the 1mM of IPTG induction. Relative fluorescence intensity was calculated with normalization of OD600 value. The relative fluorescence intensity of S2 control group was set arbitrarily at 1.0, and the levels of other groups were adjusted correspondingly. This experiment was run in three parallel reactions, and the data represent results obtained from at least three independent experiments. *0.01<p<0.05.
The results shows that the existence of GFP1 and GFP2 can observably increase the fluorescent intensity. Which then indicates that these two parts work as expected.