Difference between revisions of "Part:BBa K1689010"
Spring zhq (Talk | contribs) |
|||
| Line 4: | Line 4: | ||
Nluc-dCas9 fusion protein ORF | Nluc-dCas9 fusion protein ORF | ||
| − | A catalytically dead Cas9 (dCas9), when | + | A catalytically dead Cas9 (dCas9), when co-expressed with a guide RNA, forms a DNA recognition complex which can binds any sequence [1]. Firefly luciferase, widely used as a reporter, is split into two fragments, namely N-luc and C-luc [2]. Each fragment by itself is inactive; when two fragments are reassembled, the enzymatic activity of the original protein would be reconstituted, providing easily measurable readout. |
| − | + | Peking iGEM 2015 fused N-luc to the N-terminus of dCas9 (Figure 1). Guided by sgRNA, it binds to the target DNA. Together with another part, C-luc-dCas9 [https://parts.igem.org/wiki/index.php?title=Part:BBa_K1689008 (BBa_K1689008)]:sgRNA complex, our paired dCas9 (PC) reporter system would work to (Figure 2) to convert the sequence-specific information of pathogenic bacteria's genome (in our case, M. tuberculosis) into easily readable bioluminescence signal. | |
[[File:Peking-Nluc-dCas-2015-part-test.png|400px|]] | [[File:Peking-Nluc-dCas-2015-part-test.png|400px|]] | ||
| − | '''Figure 1. | + | '''Figure 1. Schematic cartoon of Nluc-dCas9 fusion protein.''' |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
[[File:Peking-CRISPR-Figure2.png|700px|]] | [[File:Peking-CRISPR-Figure2.png|700px|]] | ||
| − | '''Figure | + | '''Figure 2. Working mechanism of the paired dCas9 (PC) reporter system.''' |
| − | We | + | We thoroughly optimized the configuration of our PC reporter system (see [http://2015.igem.org/Team:Peking/Design/PC_Reporter Methods]). Provided that the initial binding of dCas9 to DNA depends on the protospacer adjacent motif (PAM, a short 3’ motif adjacent to target sequence), four sets of sgRNA orientation settings were tested (Figure 3a). To find how the split luciferase-dCas9 fusion strategy influences the performance of our PC reporter system, we constructed and tested C-luc-dCas9 [https://parts.igem.org/wiki/index.php?title=Part:BBa_K1689009 (BBa_K1689009)], dCas9-C-luc [https://parts.igem.org/wiki/index.php?title=Part:BBa_K1689007 (BBa_K1689007)] to pair with N-luc-dCas9 and dCas9-C-luc, respectively (Figure 3b). |
a | a | ||
| Line 36: | Line 30: | ||
| − | '''Figure | + | '''Figure 3. Thorough optimization on the configuration of our PC reporter system. ''' (a) 4 different sgRNA orientation settings. In orientation PAM-out, the pair of PAM sequences are distal from the spacer sequence, with the 5' end of the sgRNA adjacent to the spacer; in orientation PAM-in, the pair of PAM sequences are adjacent to the spacer sequence, with the 3' end of the sgRNA in proximity to the spacer; in orientation PAM-direct 1 and PAM-direct 2, one PAM sequence is adjacent to and another distal from the spacer. (b) 4 different protein fusion strategies for the integration of split luciferase fragments into dCa9. |
| − | We chose | + | We chose N-luc-dCas9/C-luc-dCas9 fusion proteins with sgRNA orientation PAM-out for the following measurement because it presents the highest signal and robustness. Then we validated its specificity and sensitivity. Specificity test was carried out to distinguish the target plasmid [https://parts.igem.org/Part:BBa_K909009 (BBa_K909009)] from different non-specific controls (Figure 4a,b,c). The results showed that the luminescence intensity using the target is significantly higher than that of non-specific controls (Figure 4d). |
[[File:Peking-CRISPR-Figure7.png|700px|]] | [[File:Peking-CRISPR-Figure7.png|700px|]] | ||
| − | '''Figure | + | '''Figure 4. Specificity test of PC reporter system.''' (a) Positive target with two sites recognized simultaneously by a pair of split luciferase-dCas9:sgRNA complexes. (b) Control with only one site recognized by "half pair". (c) Negative control with no site to be recognized. (d) Experimental data. Error bars denote s.d.; n=3. |
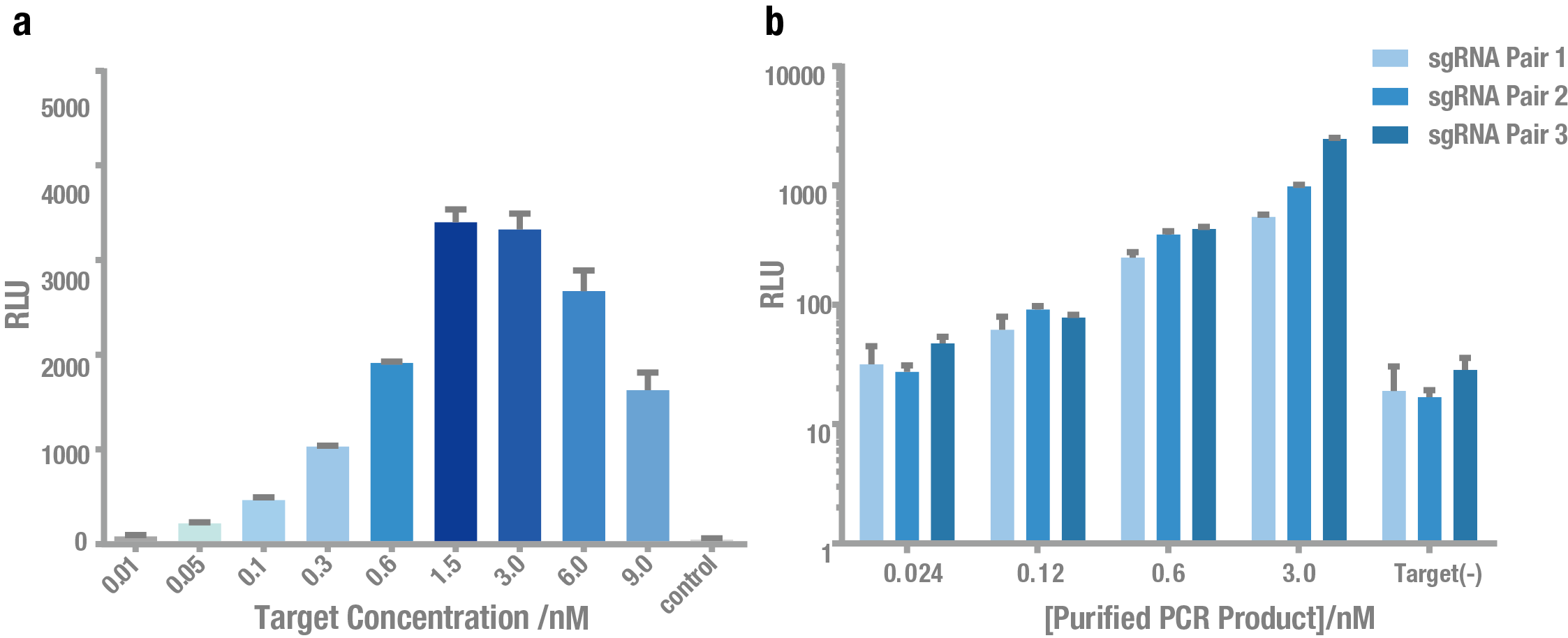
| − | Then, we tested the sensitivity of PC reporter system. As shown in Figure 5, PC reporter can still work at the concentration of ~0.1nM target for both plasmid (Figure | + | Then, we tested the sensitivity of PC reporter system. As shown in Figure 5, PC reporter can still work at the concentration of ~0.1nM target for both plasmid (Figure 5a) and crude PCR product (Figure 5b). |
[[File:Peking-CRISPR-Figure8.png|700px|]] | [[File:Peking-CRISPR-Figure8.png|700px|]] | ||
| − | '''Figure | + | '''Figure 5. Exploring the sensitivity of PC reporter using plasmid and PCR product as the target.''' (a) Bioluminescence intensity using different concentrations of plasmid as the target. (b) Bioluminescence intensity using different concentrations of crude PCR product as the target. Both showed that PC reporter was able to detect target DNA at a concentration (0.1 nM). |
==References== | ==References== | ||
| − | 1. | + | 1. Lei S. Qi, Matthew H. Larson, Luke A. Gilbert et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell, 2013, 152: 1173-1183. |
| − | 2. | + | 2. Kathryn E. Luker, Matthew C. P. Smith, et al. Kinetics of regulated protein–protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. PNAS, 2004, 101: 12288-12293. |
<!-- --> | <!-- --> | ||
Revision as of 03:12, 19 September 2015
N-luc-dCas9
Nluc-dCas9 fusion protein ORF
A catalytically dead Cas9 (dCas9), when co-expressed with a guide RNA, forms a DNA recognition complex which can binds any sequence [1]. Firefly luciferase, widely used as a reporter, is split into two fragments, namely N-luc and C-luc [2]. Each fragment by itself is inactive; when two fragments are reassembled, the enzymatic activity of the original protein would be reconstituted, providing easily measurable readout.
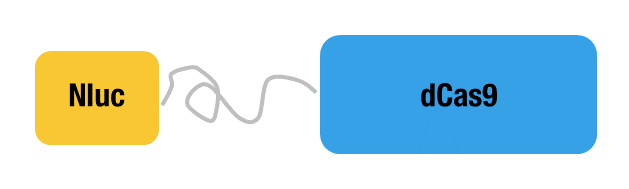
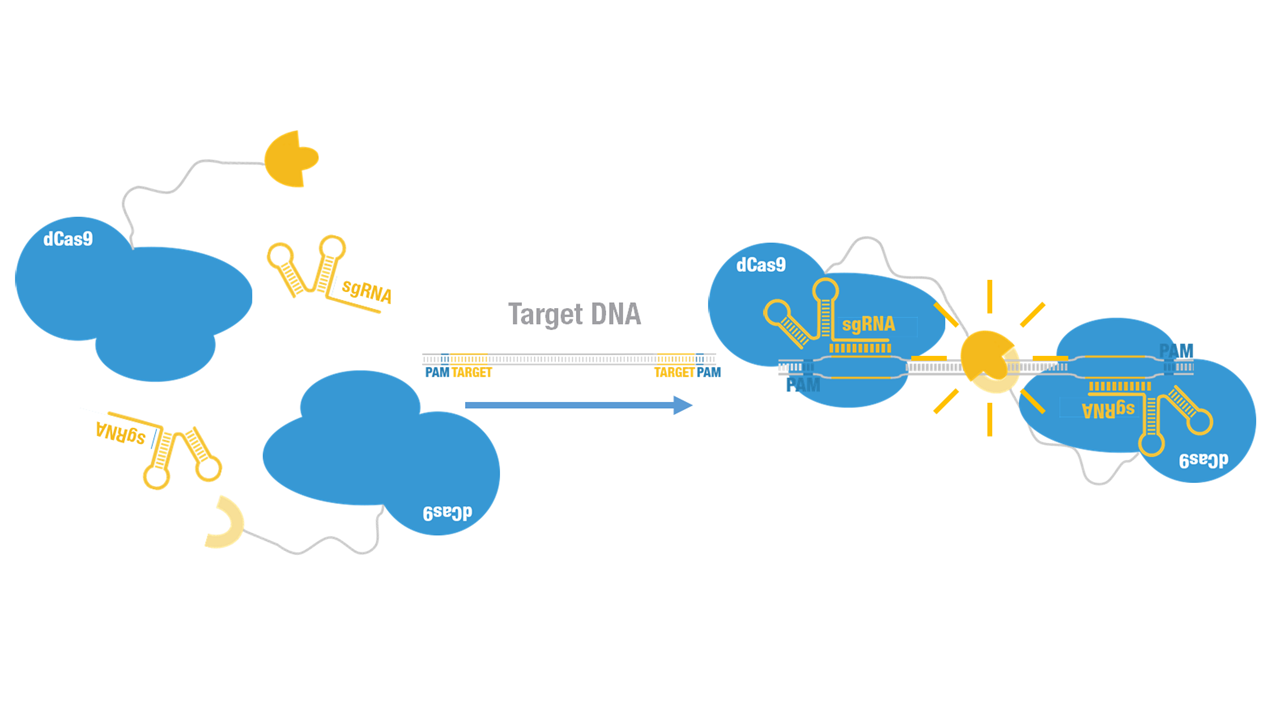
Peking iGEM 2015 fused N-luc to the N-terminus of dCas9 (Figure 1). Guided by sgRNA, it binds to the target DNA. Together with another part, C-luc-dCas9 (BBa_K1689008):sgRNA complex, our paired dCas9 (PC) reporter system would work to (Figure 2) to convert the sequence-specific information of pathogenic bacteria's genome (in our case, M. tuberculosis) into easily readable bioluminescence signal.
Figure 1. Schematic cartoon of Nluc-dCas9 fusion protein.
Figure 2. Working mechanism of the paired dCas9 (PC) reporter system.
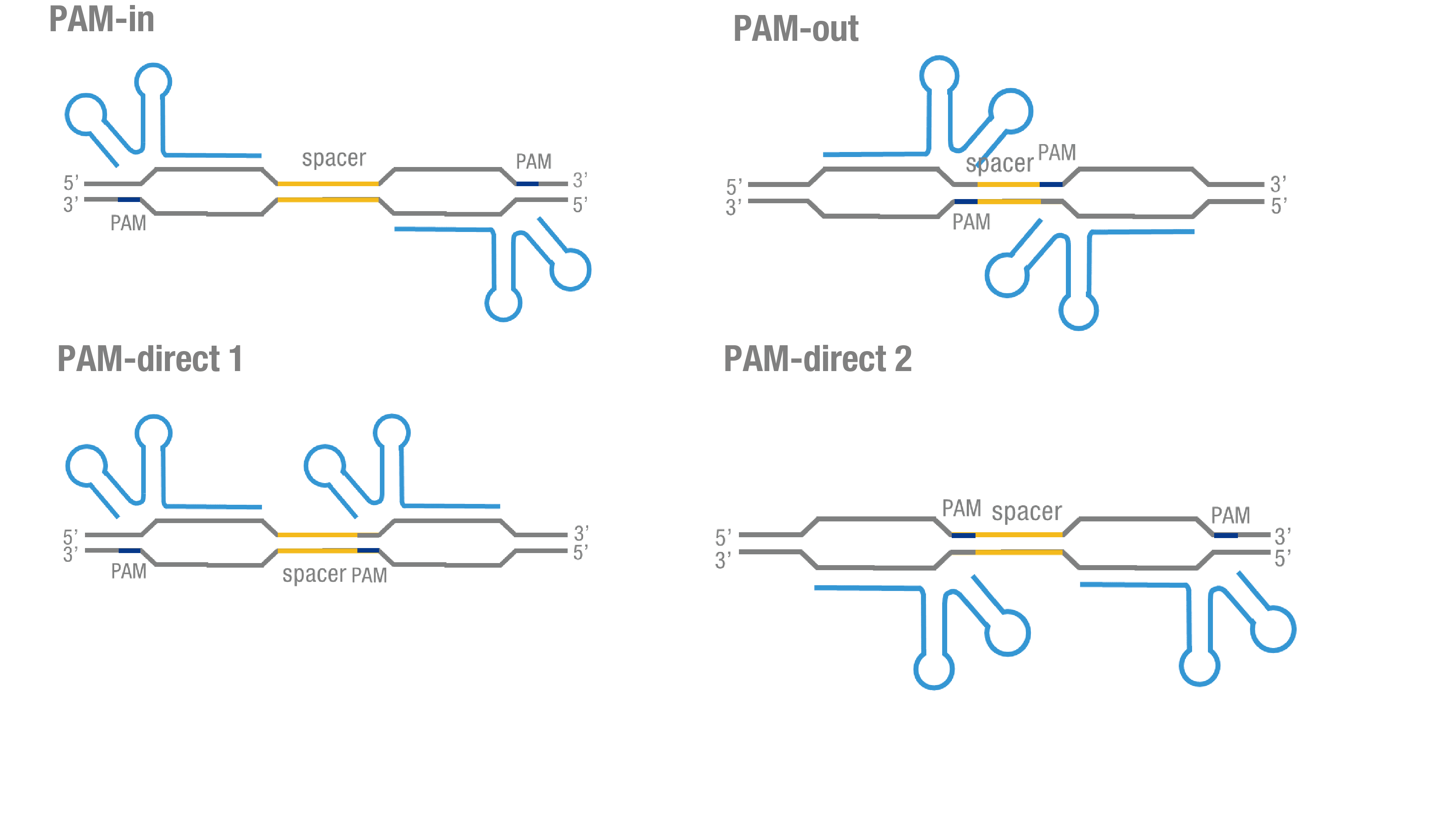
We thoroughly optimized the configuration of our PC reporter system (see [http://2015.igem.org/Team:Peking/Design/PC_Reporter Methods]). Provided that the initial binding of dCas9 to DNA depends on the protospacer adjacent motif (PAM, a short 3’ motif adjacent to target sequence), four sets of sgRNA orientation settings were tested (Figure 3a). To find how the split luciferase-dCas9 fusion strategy influences the performance of our PC reporter system, we constructed and tested C-luc-dCas9 (BBa_K1689009), dCas9-C-luc (BBa_K1689007) to pair with N-luc-dCas9 and dCas9-C-luc, respectively (Figure 3b).
a
b
Figure 3. Thorough optimization on the configuration of our PC reporter system. (a) 4 different sgRNA orientation settings. In orientation PAM-out, the pair of PAM sequences are distal from the spacer sequence, with the 5' end of the sgRNA adjacent to the spacer; in orientation PAM-in, the pair of PAM sequences are adjacent to the spacer sequence, with the 3' end of the sgRNA in proximity to the spacer; in orientation PAM-direct 1 and PAM-direct 2, one PAM sequence is adjacent to and another distal from the spacer. (b) 4 different protein fusion strategies for the integration of split luciferase fragments into dCa9.
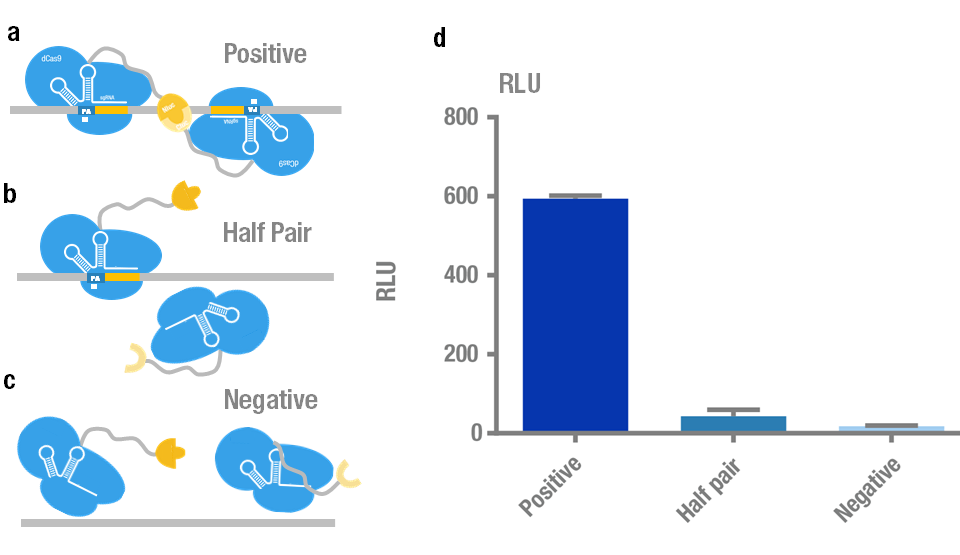
We chose N-luc-dCas9/C-luc-dCas9 fusion proteins with sgRNA orientation PAM-out for the following measurement because it presents the highest signal and robustness. Then we validated its specificity and sensitivity. Specificity test was carried out to distinguish the target plasmid (BBa_K909009) from different non-specific controls (Figure 4a,b,c). The results showed that the luminescence intensity using the target is significantly higher than that of non-specific controls (Figure 4d).
Figure 4. Specificity test of PC reporter system. (a) Positive target with two sites recognized simultaneously by a pair of split luciferase-dCas9:sgRNA complexes. (b) Control with only one site recognized by "half pair". (c) Negative control with no site to be recognized. (d) Experimental data. Error bars denote s.d.; n=3.
Then, we tested the sensitivity of PC reporter system. As shown in Figure 5, PC reporter can still work at the concentration of ~0.1nM target for both plasmid (Figure 5a) and crude PCR product (Figure 5b).
Figure 5. Exploring the sensitivity of PC reporter using plasmid and PCR product as the target. (a) Bioluminescence intensity using different concentrations of plasmid as the target. (b) Bioluminescence intensity using different concentrations of crude PCR product as the target. Both showed that PC reporter was able to detect target DNA at a concentration (0.1 nM).
References
1. Lei S. Qi, Matthew H. Larson, Luke A. Gilbert et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell, 2013, 152: 1173-1183.
2. Kathryn E. Luker, Matthew C. P. Smith, et al. Kinetics of regulated protein–protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. PNAS, 2004, 101: 12288-12293.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 2451
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 3
Illegal BamHI site found at 4730 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]