Difference between revisions of "Part:BBa K1732005"
| Line 3: | Line 3: | ||
J23100-B0034-Renilla-B0015 | J23100-B0034-Renilla-B0015 | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[File:Renilla.jpg]] | [[File:Renilla.jpg]] | ||
| Line 70: | Line 57: | ||
[3]Coelenterazine [Fact sheet]. (n.d.). Retrieved September 14, 2015, from Gold Biotechnology website: | [3]Coelenterazine [Fact sheet]. (n.d.). Retrieved September 14, 2015, from Gold Biotechnology website: | ||
https://www.goldbio.com/product/1012/coelenterazine | https://www.goldbio.com/product/1012/coelenterazine | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <!-- Add more about the biology of this part here | ||
| + | ===Usage and Biology=== | ||
| + | |||
| + | <!-- --> | ||
| + | <span class='h3bb'>Sequence and Features</span> | ||
| + | <partinfo>BBa_K1732005 SequenceAndFeatures</partinfo> | ||
| + | |||
| + | |||
| + | <!-- Uncomment this to enable Functional Parameter display | ||
| + | ===Functional Parameters=== | ||
| + | <partinfo>BBa_K1732005 parameters</partinfo> | ||
| + | <!-- --> | ||
Revision as of 20:58, 17 September 2015
J23100-B0034-Renilla-B0015
J23100-B0034-Renilla-B0015
This sequence has been codon optimized for E. coli and is used to produce optimal amounts of Renilla luciferase so that it can be used to compare the luminescence as measured by standard luminometers as well as other DIY luminometers.
pSB1C3 backbone (part:pSB1C3) with J23100 promoter (BBa_J23100) and RBS (BBa_B0034), Renilla (BBa_52008), and B0015 terminator (BBa_B0015).
Renilla Luciferase (Rluc), is a protein that emits light when oxygen is available and coelenterazine is added. Rluc can be used as a reporter to track other proteins or to monitor the activity of a promoter (iGEM 2006_Slovenia). It is a naturally produced protein from Renilla reniformis and is 36 kDa. The protein emits blue light at of 480nm [1].
Bioluminescent Mechanism of Coelenterazine
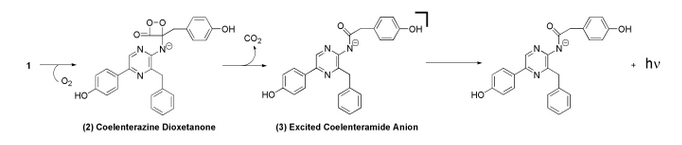
Coelenterazine is found in several aquatic organisms and is a luminescent substrate for many luciferase enzymes. For this study, Renilla Luciferase interacted with coelenterazine to determine relative light output and kinetics [3].
In the mechanism, coelenterazine reacts with oxygen to yield 1,2-dioxetane (compound 2). Subsequent loss of carbon dioxide leads to the intermediate, followed by emission of a photon [2].
The image above is the chemiluminescent mechanism of coelenterazine. The bioluminescent mechanism is similar, except that the excited state molecule in bioluminescent is phenolate anion instead of the amide anion [2].
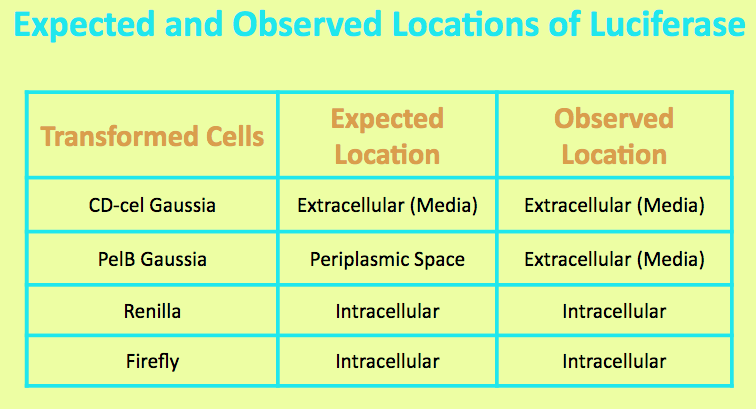
Before running experiments with Renilla Luciferase, the location of the luciferase in the media was determined by comparing the light output of overnight grown culture of Renilla cells with those of the pellet and the supernatant. The Renilla culture was spun down, and the pellet represented intracellular localization while the supernatant represented extracellular localization.
It was expected that Renilla Luciferase was localized in the cell, and the level of light output from the pellet matched that of the overnight culture and was significantly higher than that of the media. This suggested that the luciferase is located intracellularly.
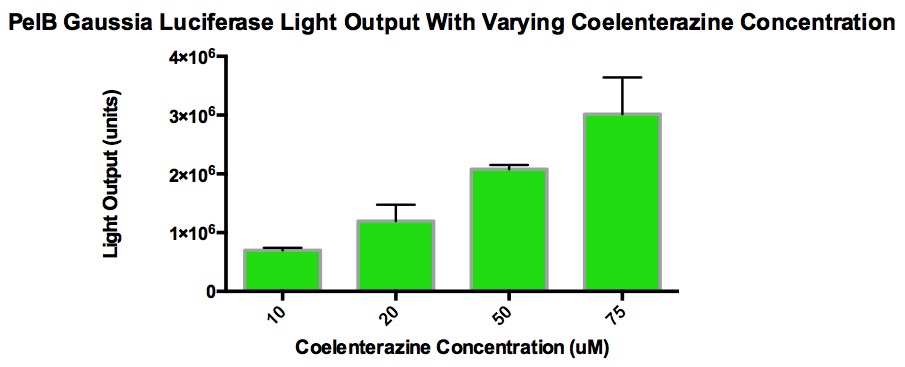
The graph shows the amount of light output produced from varying concentrations of coelenterazine when added to 100uL of Renilla cells grown overnight (5mL LB broth, 5uL Chloramphenicol, single cell colony). 10uL of coelenterazine was added and this volume stayed consistent. The overnight culture was diluted 1/10 with LB broth in order to measure the Klett OD and stay within accurate measurement range. The purpose of this is to see the effect of light output with increasing concentrations of coelenterazine. The goal was to find a concentration that plateau without maxing out the luminometer (TECAN). It was discovered that at a certain concentration, the light output stop increasing because the acidic methanol used to make the stock solution of coelenterazine began to interfere with the enzyme activity. Also, the luciferase is localized within the cell, which hinders the reaction with coelenterazine. This explains why high concentration of coelenterazine produces lower amount of light.
The two types of competent cells used were Mach and Top10 cells. Mach cells are one of the fastest growing competent strain and Top10 cells also have transformation and cloning efficiency. Both are able to replicate high number of plasmids at stable levels.
From the graphs, it can be determined that higher concentrations of coelenterazine allows for a higher light output. However, the decay rate is not as significant as that of PelB Gaussia at higher concentration of coelenterazine. In addition, the plateau level steadies at a higher light output when given higher concentrations of coelenterazine. It is also important to note that the plateau occurs quickly.
References
[1]What are some of the differences between Renilla luciferase and firefly luciferase? [Fact sheet].
(n.d.). Retrieved September 14, 2015, from Promega website: http://www.promega.com/resources/
pubhub/enotes/what-are-some-of-the-differences-between-renilla-luciferase-and-firefly-luciferase/
[2]Gonzalez, V. M., Jr. (2007). Synthesis, Luminescence, and Applications of Coelenterazine and its
Analogs. University of Illinois Urbana-Champaign.
[3]Coelenterazine [Fact sheet]. (n.d.). Retrieved September 14, 2015, from Gold Biotechnology website:
https://www.goldbio.com/product/1012/coelenterazine
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 832
Illegal XhoI site found at 641 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 265
- 1000COMPATIBLE WITH RFC[1000]