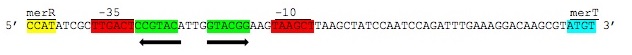Difference between revisions of "Part:BBa K1420004"
(→Molecular Function and Mechanism) |
(→Molecular Function and Mechanism) |
||
| Line 24: | Line 24: | ||
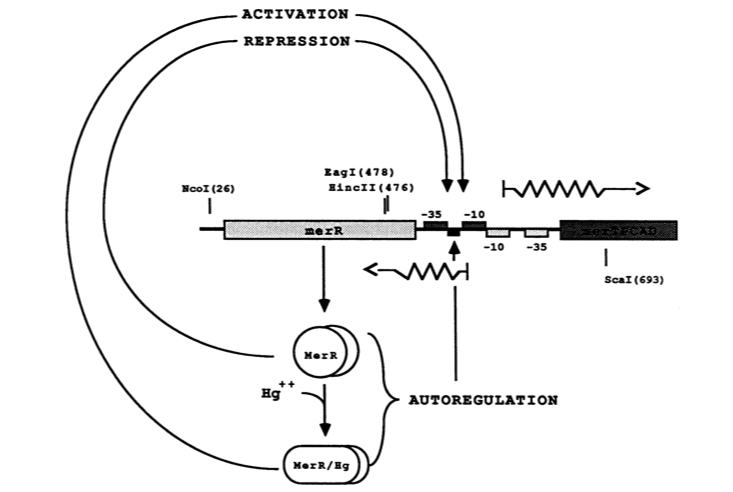
''Figure 2.'' The figure above shows MerR diamers both in the presence and absence of Hg(II). In both cases the diamer will bind the regulatory intergenic sequence but only when the diamer has undergone a conformational change by binding Hg(II) will transcription be induced, both of merR and the entire operon. Source: Park, S. J., Wireman, J. & Summers, A. O. Genetic analysis of the Tn21 mer operator-promoter. J. Bacteriol. 174, 2160 (1992). | ''Figure 2.'' The figure above shows MerR diamers both in the presence and absence of Hg(II). In both cases the diamer will bind the regulatory intergenic sequence but only when the diamer has undergone a conformational change by binding Hg(II) will transcription be induced, both of merR and the entire operon. Source: Park, S. J., Wireman, J. & Summers, A. O. Genetic analysis of the Tn21 mer operator-promoter. J. Bacteriol. 174, 2160 (1992). | ||
| − | |||
| − | |||
<p>[[File:merR_sequence.JPEG ]]</p> | <p>[[File:merR_sequence.JPEG ]]</p> | ||
| − | + | ''Figure 3.'' Intergenic sequences used to regulate the ''mer'' operon. The displayed region is between the ''merR'' and ''merT'' genes containing the -35 and -10 elements of the PmerT inducible promoter highlighted above in red. Between these elements are contained the two palindromic MerR binding sites highlighted in green. Hg(II) bound MerR homodimers bind this region to induce conformational change in the promoter region to allow for DNA polymerase binding and transcription of the ''mer'' operon. (Image adapted from Summers et al 1992) | |
[[File:merR_3.JPEG ]] | [[File:merR_3.JPEG ]] | ||
Revision as of 01:45, 18 October 2014
merR family transcriptional regulator, regulatory protein for mer operon
Summary
• merR, located upstream of the rest of the mer operon mercury resistance genes, serves to regulate the mer operon by activating transcription in the presence of Hg(II) and acting as a weak repressor in the absence of Hg(II).
• The MerR protein contains a C-terminal effector-binding region that recognizes ionic mercury, forming a homodimer upon binding to Hg(II).
• The effector binding region of MerR family proteins can vary allowing for great diversity in MerR-like promoters that can respond to a variety of heavy metals as well as antibiotics and oxidative stress.
Overview
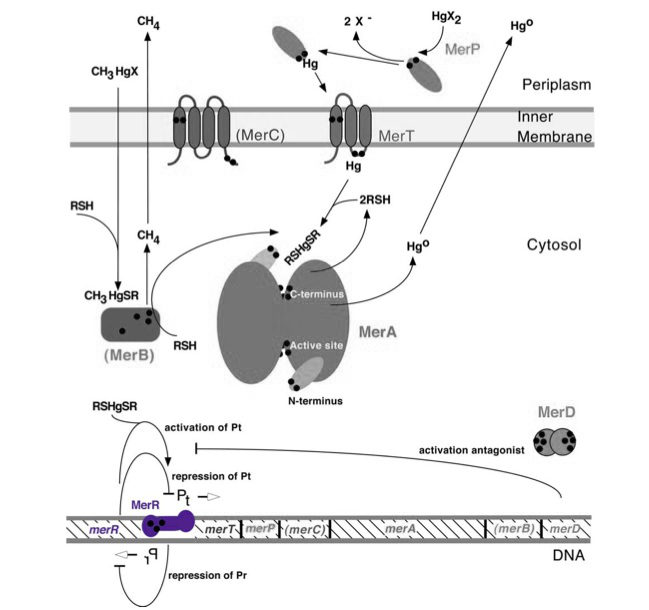
merR, located upstream of the rest of the mer operon mercury resistance genes (see the bottom of Figure 1), serves to regulate the mer operon by activating transcription in the presence of Hg(II) and acting as a weak repressor in the absence of Hg(II). The mer operon is a set of genes that function in synchrony to convey mercury resistance to bacterial cells. For our purposes the operon can be used to bioremediate methylmercury by converting it to its less toxic ionic form, Hg(II), and then into volatile Hg(0). While variations in the operon exist, with not all genes being present in all organisms and some having extra genes, below is given a general schematic of how the proteins encoded by the operon function to convey mercury resistance to the cell.
Figure 1. The figure of the mer operon above gives both the key genes and proteins involved in mercury resistance. Black dots in the figure refer to cysteine residues, which may be key in binding mercuric compounds. MerR, highlighted in purple, is the key regulatory protein of the operon, inducing transcription in the presence of ionic mercury. MerT is an inner membrane protein that functions in conjunction with MerP to transport ionic mercury into the cytosol. MerC is a similar inner membrane protein that may not always be necessary for proper functioning of the operon. MerA may be considered the heart of the mercury resistance genes as it converts ionic mercury to the volatile Hg(0) form. For our purposes of bioremediation the function of MerB, which catalyzes the conversion of methylmercury to ionic mercury, is also key. Finally MerD is a putative antagonist of MerR that may also help to regulate the operon. Source: Barkay, T., Miller, S. M. & Summers, A. O. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 27, 355-384 (2003).
Molecular Function and Mechanism
The MerR protein contains a C-terminal effector-binding region that recognizes ionic mercury. Upon binding Hg(II) a MerR homodimer forms. The protein’s N-terminal helix-turn-helix DNA binding domain can now bind the palindromic MerR binding sequence located between the -35 and -10 elements of the PmerT inducible promoter. Figure 2 gives a generalized schematic of the regulatory function of the MerR protein, both for translation of merR and the entire operon. Figure 3 gives a more detailed outline of the intergenic sequence involved in this conformational change. The promoter has an unusually long spacer sequence of 19 bp that only allows for weak ribosome binding. The MerR/Hg(II) dimer structurally alters the promoter upon binding, allowing for tight DNA polymerase binding and transcription of the downstream genes (Figure 4). It should be noted that the effector binding region of MerR family proteins can vary allowing for great diversity in MerR-like promoters that can respond to a variety of heavy metals as well as antibiotics and oxidative stress. This variable nature of MerR family proteins makes them a valuable tool for various heavy metal detection and bioremediation.
Figure 2. The figure above shows MerR diamers both in the presence and absence of Hg(II). In both cases the diamer will bind the regulatory intergenic sequence but only when the diamer has undergone a conformational change by binding Hg(II) will transcription be induced, both of merR and the entire operon. Source: Park, S. J., Wireman, J. & Summers, A. O. Genetic analysis of the Tn21 mer operator-promoter. J. Bacteriol. 174, 2160 (1992).
Figure 3. Intergenic sequences used to regulate the mer operon. The displayed region is between the merR and merT genes containing the -35 and -10 elements of the PmerT inducible promoter highlighted above in red. Between these elements are contained the two palindromic MerR binding sites highlighted in green. Hg(II) bound MerR homodimers bind this region to induce conformational change in the promoter region to allow for DNA polymerase binding and transcription of the mer operon. (Image adapted from Summers et al 1992)
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]