Difference between revisions of "Part:BBa K1438025"
(→jbfs_mil_Ferritin expression device) |
(→An optimized human ferritin protein) |
||
| Line 4: | Line 4: | ||
=== An optimized human ferritin protein === | === An optimized human ferritin protein === | ||
| − | Constructing an optimized human ferritin BioBrick | + | ====Constructing an optimized human ferritin BioBrick==== |
| + | |||
| + | The basic parts (human ferritin light and heavy chain) were provided by Chris Wintersinger from the iGEM Calgary Team 2013. Thanks again for sending us your part <partinfo>BBa_K1189018</partinfo>. | ||
| + | |||
In general ferritins are found in all kingdoms of life and in many different cells of multicellular organisms. They fulfill manifold tasks synthesize iron concentrates required for cells to make cofactors of iron proteins (heme, FeS, mono and diiron) as well as caged ferritin Fe2O3*H2O is acting as an antioxidant crucial for bio metabolism of proteins [1]. Human Ferritin (huferritin) consits of two different domains, light and heavy chain. (See source part <partinfo>BBa_K1189018</partinfo> from iGEM Calgary 2013). | In general ferritins are found in all kingdoms of life and in many different cells of multicellular organisms. They fulfill manifold tasks synthesize iron concentrates required for cells to make cofactors of iron proteins (heme, FeS, mono and diiron) as well as caged ferritin Fe2O3*H2O is acting as an antioxidant crucial for bio metabolism of proteins [1]. Human Ferritin (huferritin) consits of two different domains, light and heavy chain. (See source part <partinfo>BBa_K1189018</partinfo> from iGEM Calgary 2013). | ||
The presented plasmid DNA construct JBFS_Mil_Ferritin is an improvement of the existing part <partinfo>BBa_K1438022</partinfo> from our iGEM Team Berlin 2014 is a standardized pQE80L plasmid backbone containing human ferritin. | The presented plasmid DNA construct JBFS_Mil_Ferritin is an improvement of the existing part <partinfo>BBa_K1438022</partinfo> from our iGEM Team Berlin 2014 is a standardized pQE80L plasmid backbone containing human ferritin. | ||
Latest revision as of 23:14, 17 October 2014
jbfs_mil_Ferritin Expression Device
An optimized human ferritin protein
Constructing an optimized human ferritin BioBrick
The basic parts (human ferritin light and heavy chain) were provided by Chris Wintersinger from the iGEM Calgary Team 2013. Thanks again for sending us your part BBa_K1189018.
In general ferritins are found in all kingdoms of life and in many different cells of multicellular organisms. They fulfill manifold tasks synthesize iron concentrates required for cells to make cofactors of iron proteins (heme, FeS, mono and diiron) as well as caged ferritin Fe2O3*H2O is acting as an antioxidant crucial for bio metabolism of proteins [1]. Human Ferritin (huferritin) consits of two different domains, light and heavy chain. (See source part BBa_K1189018 from iGEM Calgary 2013). The presented plasmid DNA construct JBFS_Mil_Ferritin is an improvement of the existing part BBa_K1438022 from our iGEM Team Berlin 2014 is a standardized pQE80L plasmid backbone containing human ferritin. We created this construct according to a paper published 2010 in Journal of Biological Chemistry (JBC) [3], they presented different compositions of Ferritin made of light (L) and heavy (H) chain, linked with an Glycine-Serine - construct (GS-Linker,*). “We found that the L*H chimera exhibits significantly enhanced iron-loading ability [...] compared to wild-type ferritin [3].“ Therefore it was necessary to combine different methods used in standard molecular biology, polymerase chain reaction (PCR) for amplification of single light and heavy chain. Assembly PCR for combination of amplified light and heavy chain containing GS-Linker sequence and furthermore ligation and transformation [2,3]. Currently our Team is characterizing the optimzed ferritin construct against different terms and conditions e.g. supplementation of iron in media, to proof a possible magnetization of Escherichia coli.
References: [1] Ferritins for Chemistry and for Life; Coord Chem Rev. 2013 Jan 15;257(2):579-586. Epub 2012 May 18., Theil EC1, Behera RK, Tosha T. [2] Improved Coexpression and Multiassembly Properties of Recombinant Human Ferritin Subunits in Escherichia coli; J. Microbiol. Biotechnol. (2008), 18(5), 926–932; Lee, Jung-Lim, Robert E. Levin, and Hae-Yeong Kim [3] Design and characterization of a chimeric ferritin with enhanced iron loading and transverse NMR relaxation rate; J Biol Inorg Chem. 2010 Aug;15(6):957-65. doi: 10.1007/s00775-010-0657-7. Epub 2010 Apr 17.; Iordanova B1, Robison CS, Ahrens ET.
Usage and Biology
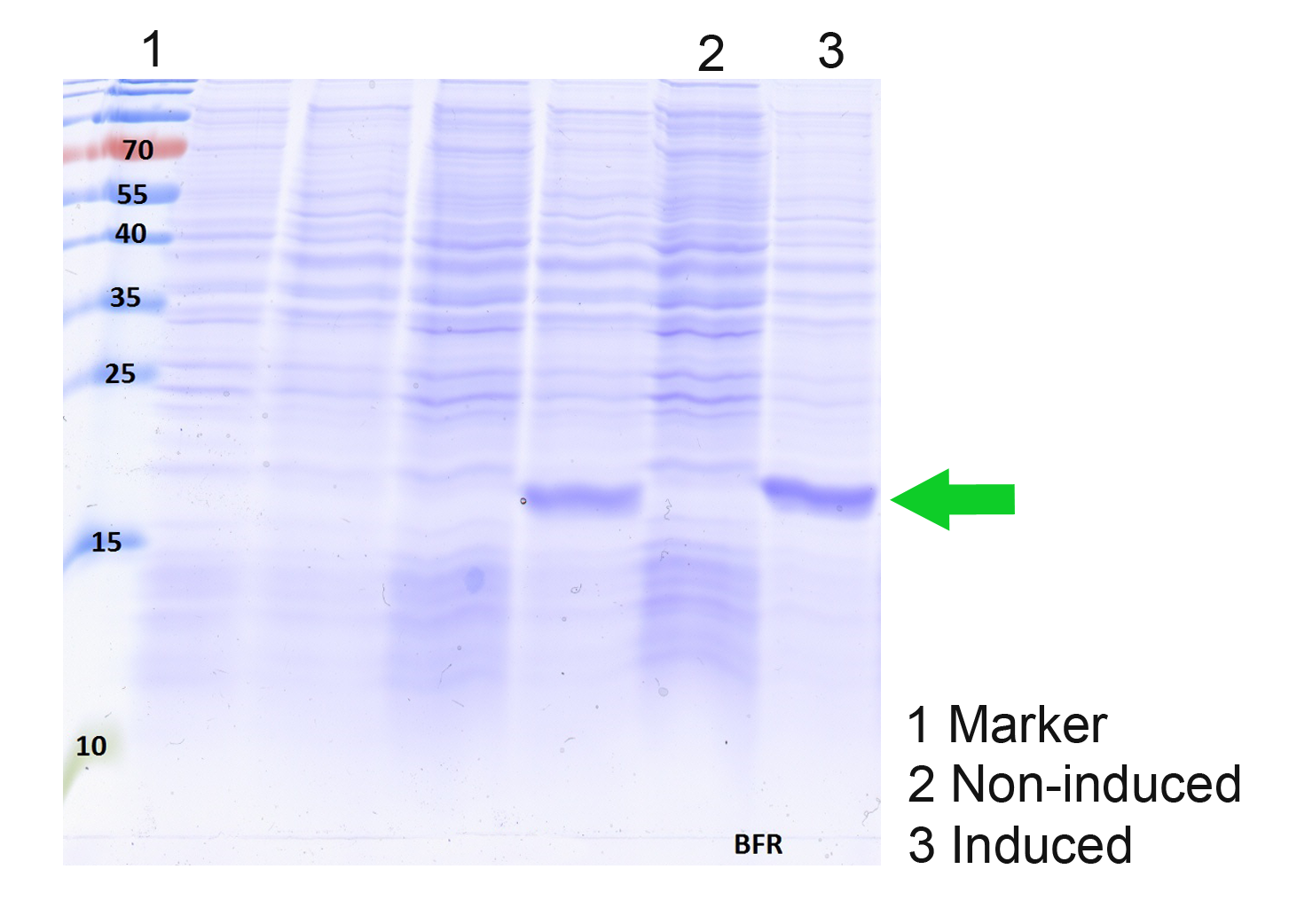
SDS PAGE
We succesfully expressed jbfs_mil_ferritin in E. coli DH10b using our BFR expression Device BBa_K1438025
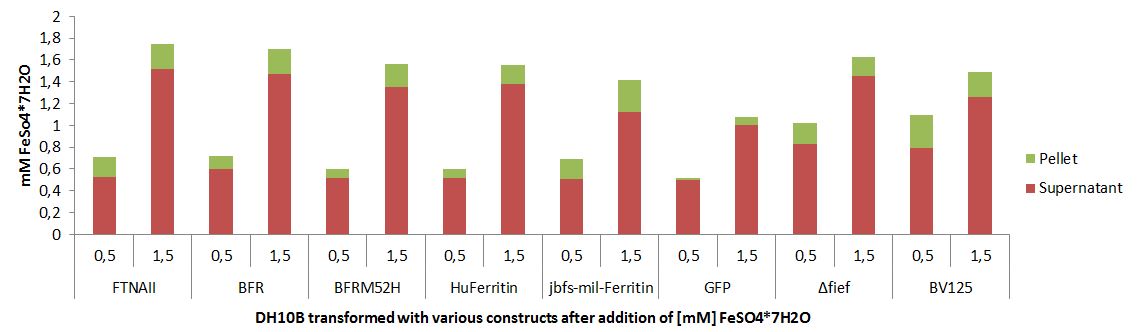
Quantification of jbfs_mil_ferritin mediated iron capacity increase
More iron can be stored inside of a cell that is overexpressing jbfs_mil_ferritin.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 88
Illegal XbaI site found at 2291
Illegal XbaI site found at 3581 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 88
Illegal NheI site found at 1435 - 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 88
Illegal BamHI site found at 145
Illegal XhoI site found at 1 - 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 88
Illegal XbaI site found at 2291
Illegal XbaI site found at 3581 - 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 88
Illegal XbaI site found at 2291
Illegal XbaI site found at 3581 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 541
Illegal BsaI.rc site found at 4954
Illegal SapI site found at 3871