Difference between revisions of "Part:BBa K1477014:Experience"
Jcampbell188 (Talk | contribs) (→Applications of BBa_K1477014) |
Geotwaddle (Talk | contribs) (→Applications of BBa_K1477014) |
||
| Line 4: | Line 4: | ||
how you used this part and how it worked out. | how you used this part and how it worked out. | ||
| − | === | + | ===Characterization and Application of BBa_K1477014=== |
| − | + | [[File:Electrophoresis_of_BBa_K1477014.png]] | |
<p> | <p> | ||
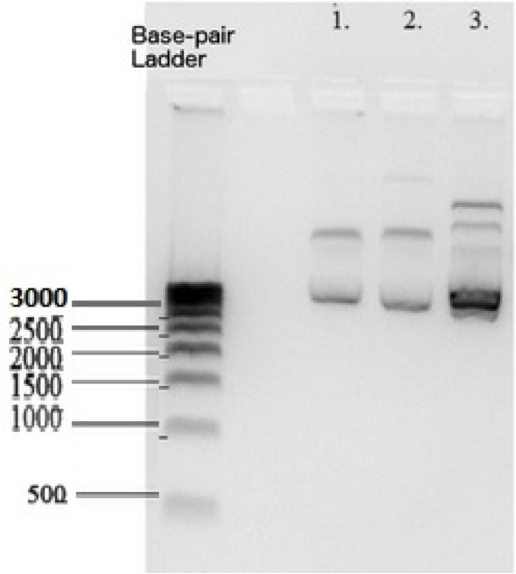
| − | According to the Biorad 500 basepair DNA ladder that we used , K1477014 measures at ~2700 base pairs | + | According to the Biorad 500 basepair DNA ladder that we used , K1477014 measures at ~2700 base pairs within pSB1C3 backbone. This matches the sum of the basepairs in individual parts and the backbone. Plasmid DNA isolated from 3 subclones.</P> |
http://i.imgur.com/kwgTE7u.png | http://i.imgur.com/kwgTE7u.png | ||
<p> | <p> | ||
| − | + | Top10 cells transformed with BBa_K1477014 form blue-green colonies on agar containing X-gal, IPTG, and selective antibiotic confirming the production of a functional β-gal alpha polypeptide and alpha complementation occuring in situ. (2 subclones) | |
| + | </P> | ||
| + | <p> | ||
| + | [[File:Extracellular_alpha_complementation.png]] | ||
| + | FUNCTIONAL Bgal ALPHA POLYPEPTIDE RELEASED BY CELL LYSIS. This graph represents the results for extracellular alpha complementation of beta galactosidase activity following lytic coliphage liberation of the fragments from transformed cells. To obtain only alpha fragments form BBa_K1477014 we grew the cells on glucose which suppresses the production of the omega polypeptide from the Lac promoter that controls the expression of the delta M15 mutation in the Top 10 host cells. In this assay, we either stimulated beta-gal or omega polypeptide expression with IPTG or suppressed it with glucose. After stimulation or suppression, T7 bacteriophage was added and the cells were incubated for 2 hrs to insure significant cell lysis. Released beta-gal enzyme or beta-gal polypeptide fragments were recovered with SpinX 0.22 micron filter units. Filtrates were combined with ONPG and absorbance was measured at 415 nanometers. | ||
| + | |||
| + | Beta-gal activity was recovered from both E. coli C and K104 stimulated with IPTG and this activity was surpressed by glucose. In the case of K104, the suppression of beta-gal activity was because of the inhibition of the production of the omega polypeptide. The alpha polypeptide in BBa_K1477014 is always produced because it is on a constitutive promoter. | ||
| + | |||
| + | To test whether alpha complementation could occur extracellularly, a cell filtrate of IPTG Top 10 cells were combined with a cell filtrate from K014 cells grown on glucose. β-gal activity unfortunately was not detected in this assay. However we found that when the filtrates were combined and incubated at 4C for 18hrs β-gal activity was detectable suggesting that extracellular complementation had occurred at a slow rate. | ||
</P> | </P> | ||
Revision as of 16:29, 2 November 2014
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Characterization and Application of BBa_K1477014
According to the Biorad 500 basepair DNA ladder that we used , K1477014 measures at ~2700 base pairs within pSB1C3 backbone. This matches the sum of the basepairs in individual parts and the backbone. Plasmid DNA isolated from 3 subclones.
http://i.imgur.com/kwgTE7u.png
Top10 cells transformed with BBa_K1477014 form blue-green colonies on agar containing X-gal, IPTG, and selective antibiotic confirming the production of a functional β-gal alpha polypeptide and alpha complementation occuring in situ. (2 subclones)
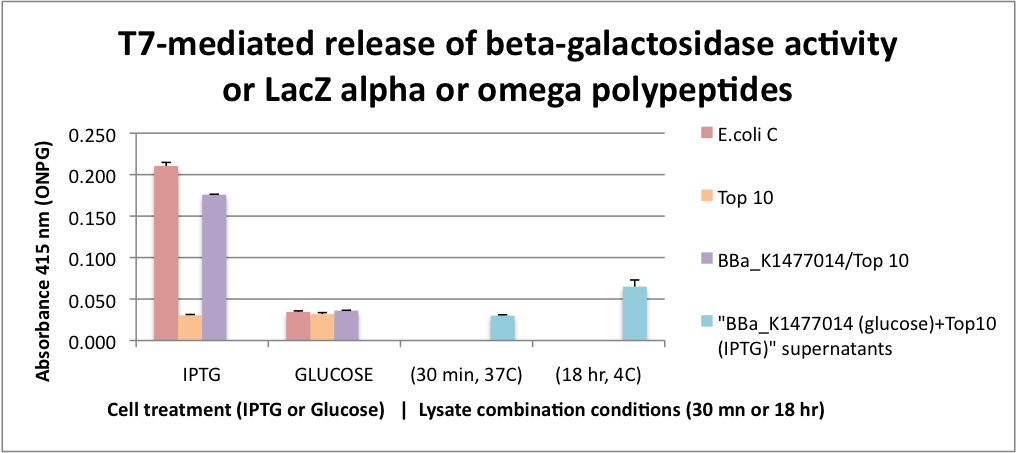 FUNCTIONAL Bgal ALPHA POLYPEPTIDE RELEASED BY CELL LYSIS. This graph represents the results for extracellular alpha complementation of beta galactosidase activity following lytic coliphage liberation of the fragments from transformed cells. To obtain only alpha fragments form BBa_K1477014 we grew the cells on glucose which suppresses the production of the omega polypeptide from the Lac promoter that controls the expression of the delta M15 mutation in the Top 10 host cells. In this assay, we either stimulated beta-gal or omega polypeptide expression with IPTG or suppressed it with glucose. After stimulation or suppression, T7 bacteriophage was added and the cells were incubated for 2 hrs to insure significant cell lysis. Released beta-gal enzyme or beta-gal polypeptide fragments were recovered with SpinX 0.22 micron filter units. Filtrates were combined with ONPG and absorbance was measured at 415 nanometers.
Beta-gal activity was recovered from both E. coli C and K104 stimulated with IPTG and this activity was surpressed by glucose. In the case of K104, the suppression of beta-gal activity was because of the inhibition of the production of the omega polypeptide. The alpha polypeptide in BBa_K1477014 is always produced because it is on a constitutive promoter.
To test whether alpha complementation could occur extracellularly, a cell filtrate of IPTG Top 10 cells were combined with a cell filtrate from K014 cells grown on glucose. β-gal activity unfortunately was not detected in this assay. However we found that when the filtrates were combined and incubated at 4C for 18hrs β-gal activity was detectable suggesting that extracellular complementation had occurred at a slow rate.
FUNCTIONAL Bgal ALPHA POLYPEPTIDE RELEASED BY CELL LYSIS. This graph represents the results for extracellular alpha complementation of beta galactosidase activity following lytic coliphage liberation of the fragments from transformed cells. To obtain only alpha fragments form BBa_K1477014 we grew the cells on glucose which suppresses the production of the omega polypeptide from the Lac promoter that controls the expression of the delta M15 mutation in the Top 10 host cells. In this assay, we either stimulated beta-gal or omega polypeptide expression with IPTG or suppressed it with glucose. After stimulation or suppression, T7 bacteriophage was added and the cells were incubated for 2 hrs to insure significant cell lysis. Released beta-gal enzyme or beta-gal polypeptide fragments were recovered with SpinX 0.22 micron filter units. Filtrates were combined with ONPG and absorbance was measured at 415 nanometers.
Beta-gal activity was recovered from both E. coli C and K104 stimulated with IPTG and this activity was surpressed by glucose. In the case of K104, the suppression of beta-gal activity was because of the inhibition of the production of the omega polypeptide. The alpha polypeptide in BBa_K1477014 is always produced because it is on a constitutive promoter.
To test whether alpha complementation could occur extracellularly, a cell filtrate of IPTG Top 10 cells were combined with a cell filtrate from K014 cells grown on glucose. β-gal activity unfortunately was not detected in this assay. However we found that when the filtrates were combined and incubated at 4C for 18hrs β-gal activity was detectable suggesting that extracellular complementation had occurred at a slow rate.
User Reviews
UNIQdc8f121a625cd0c0-partinfo-00000000-QINU UNIQdc8f121a625cd0c0-partinfo-00000001-QINU