Difference between revisions of "Part:BBa K1420002"
| Line 5: | Line 5: | ||
<p></p> | <p></p> | ||
<p></p> | <p></p> | ||
| − | <p>The ''merB'' gene is often found immediately downstream of ''merA'', and is essential for the detoxification and bioremediation of organic toxic mercury compounds in congruence with ''merA''. The ''merB'' protein is a lyase that catalyzes the breaking of carbon-mercury bonds through protonolysis of toxic mercury compounds, such as methylmercury ( | + | <p>The ''merB'' gene is often found immediately downstream of ''merA'', and is essential for the detoxification and bioremediation of organic toxic mercury compounds in congruence with ''merA''. The ''merB'' protein is a lyase that catalyzes the breaking of carbon-mercury bonds through protonolysis of toxic mercury compounds, such as methylmercury (''Scheme 1''). This produces the less toxic and less mobile Hg<sup>2+</sup> which is then completely volatilized to Hg<sup>0</sup> when acted upon the enzyme ''merA''.</p> |
<b>Structure and Mechanism</b> | <b>Structure and Mechanism</b> | ||
| Line 13: | Line 13: | ||
[[File:MerB_mechanism2.JPG ]] | [[File:MerB_mechanism2.JPG ]] | ||
<p></p> | <p></p> | ||
| − | <p>Enzyme MerB forms a dimer by a 2-fold pseudosymmetry around the depicted rotation axis in | + | <p>Enzyme MerB forms a dimer by a 2-fold pseudosymmetry around the depicted rotation axis in ''Figure 1''. The two subunits are color coded from blue to green distinguishing the amino-terminal end of the protein from the carboxy-terminal, respectively. Active site residues are color coded as well using van der Waals sphere representation: residues Cys-96 and Cys-159 are in yellow, and residue Asp-99 is in red. The structure of mercury-bound MerB and free MerB is extremely similar with the exception of the mercury ion. The mercury is bound to MerB by two sulfurs from the Cys-96 and Cys-159. An oxygen from a water molecule is also involved, binding to the mercury atom. On the other hand, the active site residues for the free MerB are completely inaccessible.</p> |
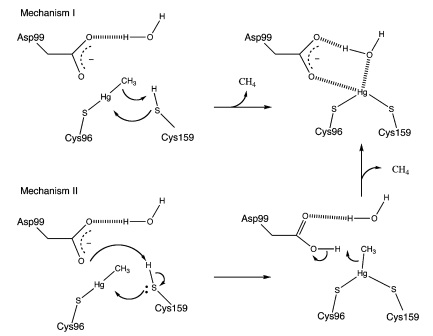
| − | <p>MerB can catalyze the protonolysis of carbon mercury bonds for a number of organomercurials, accelerating the rate of this reaction up to 10<sup>7</sup> times the rate of spontaneous abiotic decay. | + | <p>MerB can catalyze the protonolysis of carbon mercury bonds for a number of organomercurials, accelerating the rate of this reaction up to 10<sup>7</sup> times the rate of spontaneous abiotic decay. Two proposed mechanisms for how this is accomplished is displayed in ''Figure 2''. Both mechanisms assume that the methylmercury substrate forms an initial bond with Cys96 and that the Cys159 site is protonated. In Mechanism 1, Cys159 protonates the methyl carbon and forms a covalent bond with Hg(II). In Mechanism 2 however, Cys159 donates a proton to Asp99 before forming a bond with Hg(II). Asp99 is then responsible for protonating the methyl group. </p> |
| − | <b>Experimental Results</b> | + | <p></p> |
| − | < | + | <b>''merB'' Experimental Results</b> |
| + | <p></p> | ||
| + | <p></p> | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Revision as of 01:12, 10 October 2014
merB, Organomercurial Lyase from Serratia marcescens
Function
The merB gene is often found immediately downstream of merA, and is essential for the detoxification and bioremediation of organic toxic mercury compounds in congruence with merA. The merB protein is a lyase that catalyzes the breaking of carbon-mercury bonds through protonolysis of toxic mercury compounds, such as methylmercury (Scheme 1). This produces the less toxic and less mobile Hg2+ which is then completely volatilized to Hg0 when acted upon the enzyme merA.
Structure and Mechanism
Enzyme MerB forms a dimer by a 2-fold pseudosymmetry around the depicted rotation axis in Figure 1. The two subunits are color coded from blue to green distinguishing the amino-terminal end of the protein from the carboxy-terminal, respectively. Active site residues are color coded as well using van der Waals sphere representation: residues Cys-96 and Cys-159 are in yellow, and residue Asp-99 is in red. The structure of mercury-bound MerB and free MerB is extremely similar with the exception of the mercury ion. The mercury is bound to MerB by two sulfurs from the Cys-96 and Cys-159. An oxygen from a water molecule is also involved, binding to the mercury atom. On the other hand, the active site residues for the free MerB are completely inaccessible.
MerB can catalyze the protonolysis of carbon mercury bonds for a number of organomercurials, accelerating the rate of this reaction up to 107 times the rate of spontaneous abiotic decay. Two proposed mechanisms for how this is accomplished is displayed in Figure 2. Both mechanisms assume that the methylmercury substrate forms an initial bond with Cys96 and that the Cys159 site is protonated. In Mechanism 1, Cys159 protonates the methyl carbon and forms a covalent bond with Hg(II). In Mechanism 2 however, Cys159 donates a proton to Asp99 before forming a bond with Hg(II). Asp99 is then responsible for protonating the methyl group.
merB Experimental Results
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 415
- 1000COMPATIBLE WITH RFC[1000]