Difference between revisions of "Part:BBa K1420003"
| Line 2: | Line 2: | ||
<partinfo>BBa_K1420003 short</partinfo> | <partinfo>BBa_K1420003 short</partinfo> | ||
| + | ==Overview== | ||
| + | |||
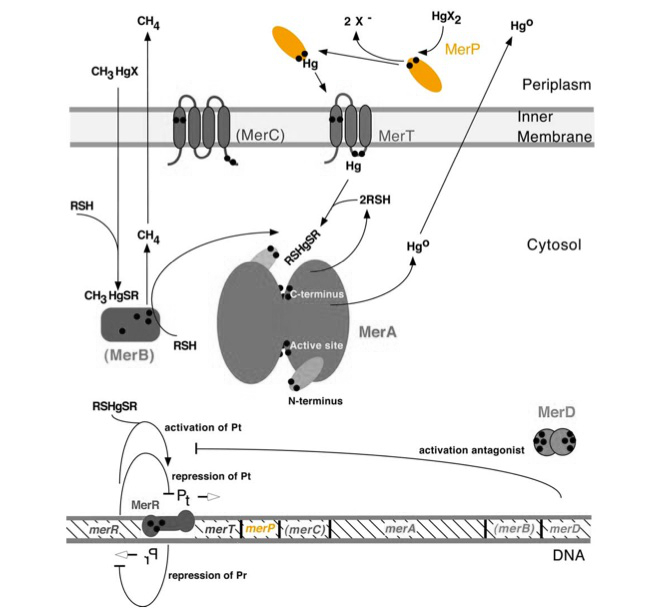
| + | <p>Periplasmic Mercury transport protein ''merP'' (0.3Kb) encodes MerP, blahblahblah. MerP and gene ''merP'' are highlighted in orange in Figure 1. The lower part of the Figure 1 shows the arrangement of ''mer'' genes in the operon, and ''merP'' is located downstream of ''merT'', another gene that encodes a mercury transport protein.</p> | ||
| + | |||
| + | [[File:MerP_gene.jpg|center|480px|x]] | ||
| + | |||
| + | <p></p> | ||
| + | Figure 1. Model of mercury resistance operon. The symbol • indicates a cysteine residue. RSH indicates cytosolic thiol redox buffers such as glutathione. Figure 1 shows the interactions of MerA with mercury compounds and other gene products of ''mer'' operon. (This figure is adapted from "Bacterial mercury resistance from atoms to ecosystems". Reference: ''T. Barkay et al''. FEMS Microbiology Reviews 27 (2003) 355-384.) | ||
Revision as of 02:54, 13 October 2014
MerP, mercuric transport protein periplasmic component
Overview
Periplasmic Mercury transport protein merP (0.3Kb) encodes MerP, blahblahblah. MerP and gene merP are highlighted in orange in Figure 1. The lower part of the Figure 1 shows the arrangement of mer genes in the operon, and merP is located downstream of merT, another gene that encodes a mercury transport protein.
Figure 1. Model of mercury resistance operon. The symbol • indicates a cysteine residue. RSH indicates cytosolic thiol redox buffers such as glutathione. Figure 1 shows the interactions of MerA with mercury compounds and other gene products of mer operon. (This figure is adapted from "Bacterial mercury resistance from atoms to ecosystems". Reference: T. Barkay et al. FEMS Microbiology Reviews 27 (2003) 355-384.)
MerP is a 72 amino acid periplasmic membrane protein. The merP protein functions as a monomer, binding a single Hg(II) ion at the two conserved cysteine residues. These Cysteines define the metal binding motif of MerP. Similar motifs are also found in other proteins that are involved in the transportation of thiophilic metal cations. The MerP Cysteine residues uptake Hg(II) ions and remove any attached ligands before passing the ion to the MerT transmembrane protein.
MerP is not essential for Hg(II) uptake, as MerT can also do much of the same work. However, there is the possibility of a Cysteine mutation on MerT which blocks Hg(II) uptake, this mutation cannot be found on MerP making it a redundant periplasmic transporter. If the MerP protein is expressed alone, it will revert to an oxidized state which is less stable than the reduced state it is found in when expressed as part of the greater Mer operon. In the periplasm, MerP competes with thiol-mediated redox proteins for Hg(II). Like periplasmic nutrient transporters, MerP is the most abundantly synthesized subunit in its operon due to its role in periplasmic Hg(II) transport.
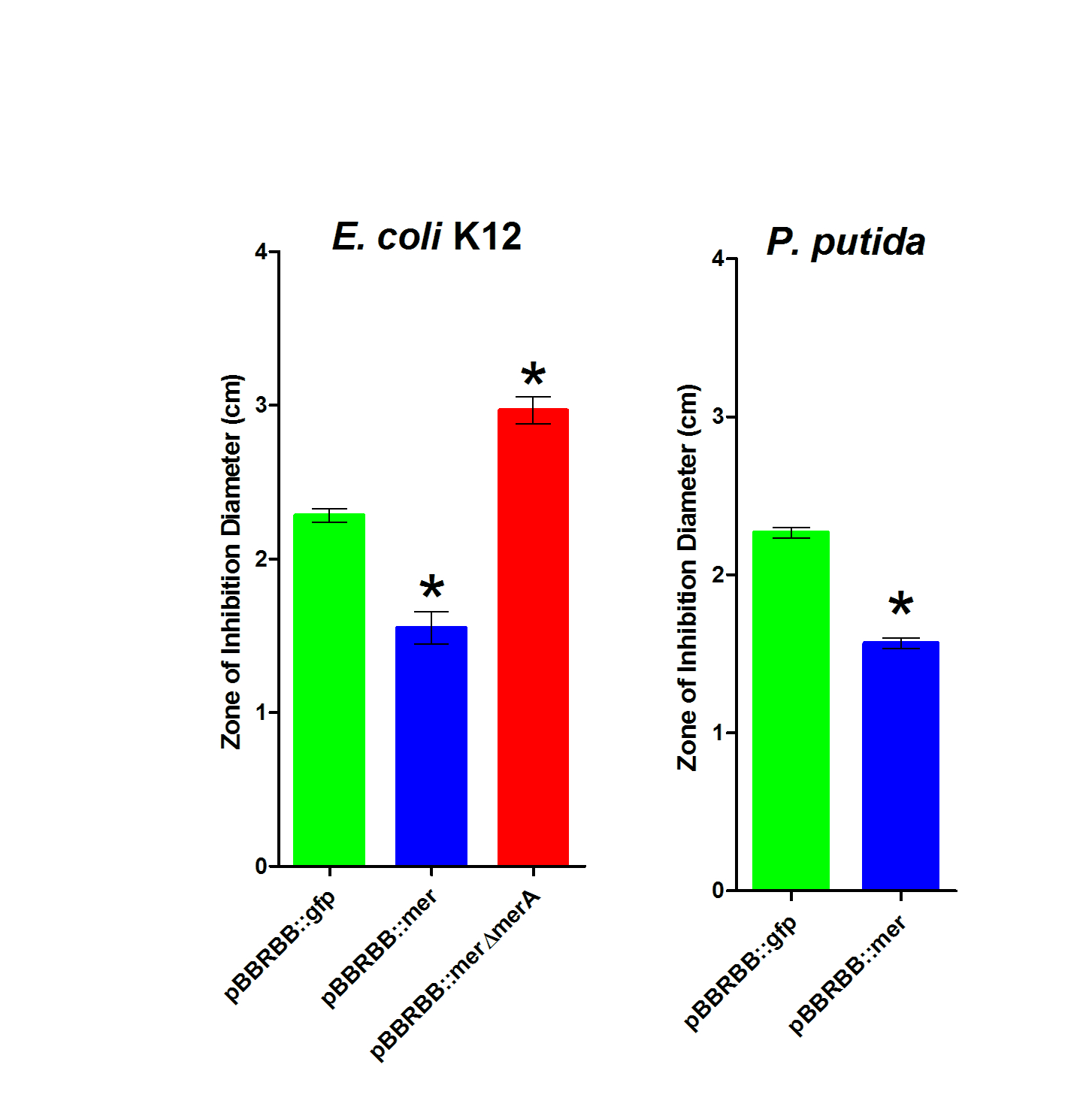
For the zone of inhibition studies, 10µL of 0.1M HgCl2 were spotted on a filter disk in the middle of an agar plate. The diameter of the Zone of Inhibition was measured in triplicate. In the linked figure, P. putida shows a smaller zone of inhibition for the strain with the Mer operon, as expected. In the E. coli K12 sample, the same relationship between the strain with the Mer operon and the empty vector was observed. In addition, vector with MerA deleted has a higher zone of inhibition, which would be expected as the bacteria would be unable to reduce Hg(II) and remove it, leading to a toxic bioaccumulation that kills the bacteria.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]