Difference between revisions of "Part:BBa K1152007"
| Line 6: | Line 6: | ||
| − | NRPS modules can be introduced by | + | NRPS modules can be introduced by Gibson ([http://www.synbio.org.uk/gibson/downloads/files/RFC57.pdf BBF RFC 57]) or CPEC cloning ([http://hdl.handle.net/1721.1/81332 BBF RFC 99]) in any desired order. NRPS modules of choice are introduced in front of an engineered IndC module containing an additional C-domain for communication with the previous module. When inserted, they are replacing the ccdB suicide gene located in front of the indigoidine module. The ccdB gene acts as a negative selection marker (death cassette) and is lost in recombined (with insert) form. During cloning the gene is replaced with an cloning efficiency close to 100 %. |
<br> | <br> | ||
[[File:Heidelberg_Registry_2007_main_fig1.png|700px|center|'''Figure 1''':]] | [[File:Heidelberg_Registry_2007_main_fig1.png|700px|center|'''Figure 1''':]] | ||
| Line 38: | Line 38: | ||
<br> | <br> | ||
===References=== | ===References=== | ||
| + | # | ||
| + | # | ||
# | # | ||
Revision as of 00:51, 29 October 2013
Helper construct for NRP-Indigoidine-tagging
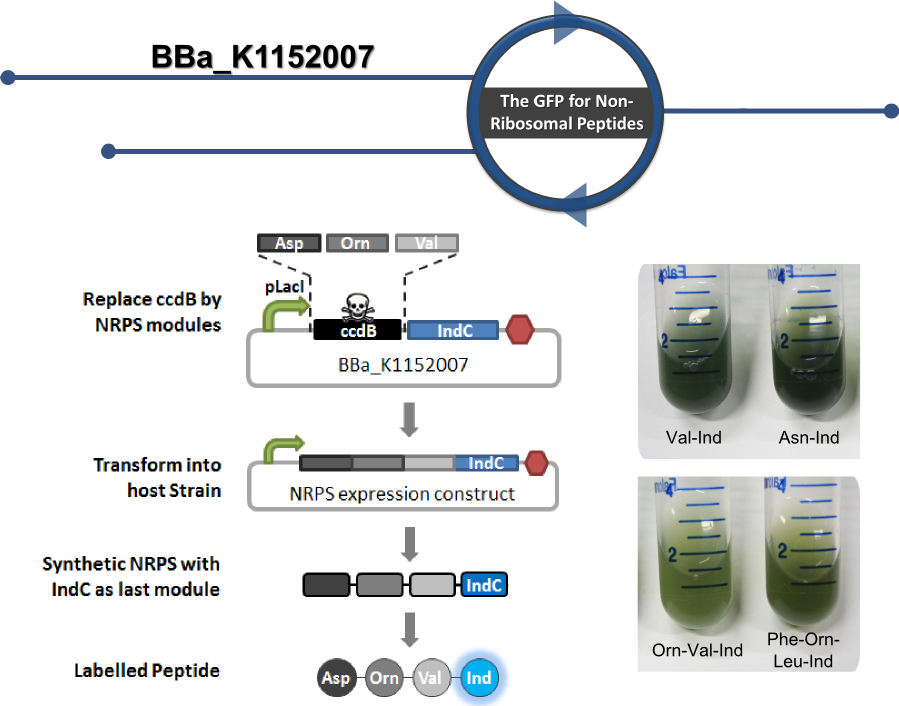
This part serves as helper plasmid for efficient cloning of custom [http://2013.igem.org/Team:Heidelberg/Project non-ribosomal peptide synthetases] (NRPSs) that enable labeling of the synthesized non-ribosomal peptides with the blue pigment indigoidine in-vivo. Peptides labelled this way can easily be purified and characterized by analytical means, since peptide production can be observed with the naked eye or quantified photometrically. Therefore, the helper-construct can be applied for high-throughput cloning of custom NRPSs for production of synthetic peptides ( [http://2013.igem.org/Team:Heidelberg/RFCs RFC 100] for details).
NRPS modules can be introduced by Gibson ([http://www.synbio.org.uk/gibson/downloads/files/RFC57.pdf BBF RFC 57]) or CPEC cloning ([http://hdl.handle.net/1721.1/81332 BBF RFC 99]) in any desired order. NRPS modules of choice are introduced in front of an engineered IndC module containing an additional C-domain for communication with the previous module. When inserted, they are replacing the ccdB suicide gene located in front of the indigoidine module. The ccdB gene acts as a negative selection marker (death cassette) and is lost in recombined (with insert) form. During cloning the gene is replaced with an cloning efficiency close to 100 %.
Note: as ccdB is replaced during cloning, only constructs bearing the desired custom NRPS sequences are successfully propagated in E. coli strains such as Top10 or DH5alpha. Thereby, false-positive-rate of clones is reduced to almost to 0 %.
Since the blue pigment indigoidine can be seen after induction with Isopropyl-β-D-thiogalactopyranosid (IPTG) by the naked eye, analytical procedures can be facilitated. Blue colonies indicate synthesis of the synthetic peptide fused to Indigoidine. A thin-layer chromatography (TLC) can be easily applied to confirm those observations.
To verify the sequence of our construct, colony PCRs, and analytical restriction digests with Pst1 and EcoRI were usually performed.
We characterized this construct by cloning different NRPS module compositions into this helper construct, leading to the production of different peptides bearing the indigoidine tag. Please refer to the experience page for charaterization of this part. Also refer to [http://hdl.handle.net/1721.1/81333 BBF RFC 100] for further details on how to engineer synthetic NRPSs.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 3061
Illegal BamHI site found at 891 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 341
Illegal SapI.rc site found at 4324
Source
- IndC was amplified from P. luminescens laumondii TT01 (DSM15139) genomic DNA.
- C(TycC2) was amplified from the tyrocidine gene cluster of Brevibacillus Parabrevis. Genebank File taken from http://www.ncbi.nlm.nih.gov/nuccore/af004835
- ccdB gene was amplified from Gateway pDONR vector (bacterial plasmide)