Difference between revisions of "Featured Parts:Fluorescent proteins"
Macampbell (Talk | contribs) |
Macampbell (Talk | contribs) |
||
| Line 1: | Line 1: | ||
==Fluorescent proteins== | ==Fluorescent proteins== | ||
| + | |||
| + | <center>[[Image:Synth_Aces.jpg]] | ||
| + | <br> | ||
| + | iGEM2005 Davidson College team logo "Synth_Aces" drawn in EYFP.</center> | ||
'''Fluorescent proteins (FP)''' are convenient was to visualize or quantify the output of a device or part. Many different FP have been cloned for use in the Registry. | '''Fluorescent proteins (FP)''' are convenient was to visualize or quantify the output of a device or part. Many different FP have been cloned for use in the Registry. | ||
| Line 5: | Line 9: | ||
<br> | <br> | ||
The first FP to be cloned, was '''green fluorescent protein (GFP)''' which as its name implies gives its biological origin (a jellyfish) its green glow. From this starting place, several investigators generated mutations that altered the spectral properties of the FP. Now we have '''enhanced yellow fluorescent protein (EYFP)''', '''cyan fluorescent protein (CFP)''' and GFP. | The first FP to be cloned, was '''green fluorescent protein (GFP)''' which as its name implies gives its biological origin (a jellyfish) its green glow. From this starting place, several investigators generated mutations that altered the spectral properties of the FP. Now we have '''enhanced yellow fluorescent protein (EYFP)''', '''cyan fluorescent protein (CFP)''' and GFP. | ||
| + | <br> | ||
| + | <br> | ||
| + | <center>[[Image:GFP.jpg]] | ||
| + | <br> | ||
| + | GFP visualized under UV light. | ||
| + | </center> | ||
<br> | <br> | ||
<br> | <br> | ||
| Line 10: | Line 20: | ||
<br> | <br> | ||
<br> | <br> | ||
| − | One more feature you should know about are '''degradation tags; LVA and AAV'''. These short amino acid tags (~11 amino acids) are added to the carboxyl terminus to increase the rate of protein degradation and thus facilitate a shorter half-life of the tagged protein. These degradation tags work in prokaryotes and allow you to “turn off” a FP in favor of another reporter state. This was used in the classic represillator of Elowitz and Leibler (2000) | + | <center>[[Image:RFP.jpg]] |
| + | <br> | ||
| + | RFP visualized in white light. Note the colonies appear red at all times and fluoresce red as well. | ||
| + | </center> | ||
| + | <br> | ||
| + | <br> | ||
| + | One more feature you should know about are '''degradation tags; LVA and AAV'''. These short amino acid tags (~11 amino acids) are added to the carboxyl terminus to increase the rate of protein degradation and thus facilitate a shorter half-life of the tagged protein. These degradation tags work in prokaryotes and allow you to “turn off” a FP in favor of another reporter state. This was used in the classic represillator of Elowitz and Leibler (2000). | ||
Revision as of 03:23, 8 April 2006
Fluorescent proteins

Fluorescent proteins (FP) are convenient was to visualize or quantify the output of a device or part. Many different FP have been cloned for use in the Registry.
The first FP to be cloned, was green fluorescent protein (GFP) which as its name implies gives its biological origin (a jellyfish) its green glow. From this starting place, several investigators generated mutations that altered the spectral properties of the FP. Now we have enhanced yellow fluorescent protein (EYFP), cyan fluorescent protein (CFP) and GFP.
GFP visualized under UV light.
Red fluorescent protein (RFP) was isolated from a very different species (a coral) and its sequence is very different from the GFP family of FP. Several derivatives of RFP have been generated, most notably mOrange and mCherry.
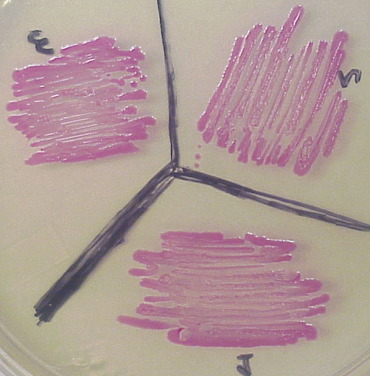
RFP visualized in white light. Note the colonies appear red at all times and fluoresce red as well.
One more feature you should know about are degradation tags; LVA and AAV. These short amino acid tags (~11 amino acids) are added to the carboxyl terminus to increase the rate of protein degradation and thus facilitate a shorter half-life of the tagged protein. These degradation tags work in prokaryotes and allow you to “turn off” a FP in favor of another reporter state. This was used in the classic represillator of Elowitz and Leibler (2000).
