Difference between revisions of "Part:BBa I13522:Experience"
(→User Reviews) |
|||
| Line 98: | Line 98: | ||
|width='60%' valign='top'| | |width='60%' valign='top'| | ||
We obtained this part from the Spring 2011 distribution plate. It expresses wonderfully in NEB10B cells from New England Biolabs. It does not express well in the fast-growing strain DH5-alpha Turbo, which we've found doesn't express transgenes very well in general. | We obtained this part from the Spring 2011 distribution plate. It expresses wonderfully in NEB10B cells from New England Biolabs. It does not express well in the fast-growing strain DH5-alpha Turbo, which we've found doesn't express transgenes very well in general. | ||
| + | |} | ||
| + | |||
| + | {|width='80%' style='border:1px solid gray' | ||
| + | |- | ||
| + | |width='10%'| | ||
| + | <partinfo>BBa_I13522 AddReview 5</partinfo> | ||
| + | <I>Wageningen UR 2014</I> | ||
| + | |width='60%' valign='top'| | ||
| + | We obtained this part from the Spring 2014 distribution plate. It doesn't express in ''E. coli'' DH5-alpha or NEB 5-alpha strains. We sequenced this part and we concluded that this parts sequence is inconsistent, missing 25 bp of the promoter sequence. Therefore, we made a twin of this part, <partinfo>BBa_K1493504</partinfo>. We did this by assembly of pTet (BBa_R0040) and GFP (BBa_I13504) using standard BioBrick assembly. Sequencing with the forward primer VF2 (5'-tgccacctgacgtctaagaa-3') confirmed the right sequence. | ||
|} | |} | ||
<!-- DON'T DELETE --><partinfo>BBa_I13522 EndReviews</partinfo> | <!-- DON'T DELETE --><partinfo>BBa_I13522 EndReviews</partinfo> | ||
Revision as of 15:04, 23 October 2014
For parts BBa_I13521 and BBa_I13522, their excitation and emission maxima were determined. BBa_B0034 was used as a negative control. These data were used later in following characterisation experiments as set parameters. The host was E. coli Top Ten in all experiments.
Meanwhile, the graph below shows the emission scan for GFP in part BBa_I13522. Since it was the best option in terms of fluorescence yield, 395 nm was set as the excitation wavelength. As it can be seen from the graph, maximum emission wavelength is 515 nm.

The characterization was performed by METU-TURKEY IGEM 2010 Team.
Thermostability Assay
For this BioBrick, the denaturation temperature was determined by heating the protein at a range of temperatures, and then measuring the fluorescence. This is a useful characterisation as it allows the selection of an appropriate reporter gene for the required temperature.
Stock solutions of GFP were prepared by extracting the protein from cell lysate, and then 50 μl aliquots of the solution were heated in a PCR thermocycler along a temperature gradient.
After two hours, 30 μl was removed from each aliquot and diluted with 170 μl of 20 mM Tris buffer to give 200 μl samples. The fluorescence of the samples was then measured on a 96-well plate. The corresponding curve was plotted on the graph below.

Results of the heat denaturation experiment. The temperature at which half of the protein is denatured is measured by observing its fluorescence (PTm50) mRFP1: 82.2°C; GFPmut3b: 61.6°C; Dendra2: 89.1°C; sfGFP: 75.0°C.
The sigmoidal curves that were calculated gave us the following function which also created the coefficient K which happens to relate to PTm50 (temperature at which half of the protein is denatured measured by looking at its fluorescence):

This characterisation was performed by the Imperial College London 2011 iGEM team.
Applications of BBa_I13522
2013 KIT-Kyoto iGEM team assessed influence of fluorescence proteins on the growth of E.coli. We used BBa_I13521, BBa_I13522 and BBa_I13600 in order to measure the growth.
We measured the turbidities of the transformants at hourly intervals and made the growth curves. We transferred the transformant prepared to 200 mL flasks. We measured the turbidities every 1 hour. The measurements were carried out for 12 hours.
Liquid LB 50mL
Sample 20μL
Ampicillin 75μL
37°C, 125rpm
Control:HST08
As the result, it was found that eCFP and mRFP have the effect of growth promotion relative to GFP.
Applications of BBa_I13522
Haynes Lab, 2012
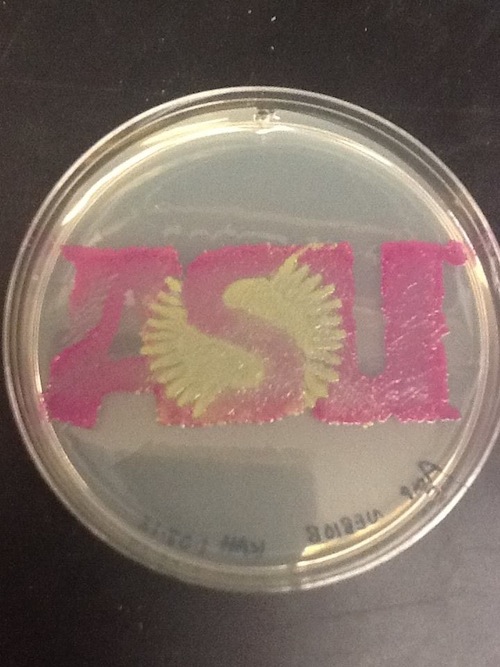
I used BBa_I13522 to demonstrate the expression of GFP in E. coli for a biomedical engineering course at Arizona State University.
- Cells: NEB10B
- Medium: LB Lennox agar, 100 ug/mL ampicillin
- The read area contains bacteria expressing RFP from BBa_J04450
iGEM team Wageningen UR
This brick was used to create GFP with a coiled coil at the N-terminus to use it as reporter for the E- and K-coil [http://2012.igem.org/Team:Wageningen_UR/Coil_system Plug and Apply sytem]
see BBa_K883704; BBa_K883705
User Reviews
UNIQdec1d2176f3a324d-partinfo-00000002-QINU
|
Antiquity |
This review comes from the old result system and indicates that this part did not work in some test. |
|
•••••
|
We obtained this part from the Spring 2011 distribution plate. It expresses wonderfully in NEB10B cells from New England Biolabs. It does not express well in the fast-growing strain DH5-alpha Turbo, which we've found doesn't express transgenes very well in general. |
|
•••••
Wageningen UR 2014 |
We obtained this part from the Spring 2014 distribution plate. It doesn't express in E. coli DH5-alpha or NEB 5-alpha strains. We sequenced this part and we concluded that this parts sequence is inconsistent, missing 25 bp of the promoter sequence. Therefore, we made a twin of this part, BBa_K1493504. We did this by assembly of pTet (BBa_R0040) and GFP (BBa_I13504) using standard BioBrick assembly. Sequencing with the forward primer VF2 (5'-tgccacctgacgtctaagaa-3') confirmed the right sequence. |
UNIQdec1d2176f3a324d-partinfo-00000007-QINU