Difference between revisions of "Part:BBa K1074000"
(→Usage and Biology) |
(→Usage and Biology) |
||
| Line 11: | Line 11: | ||
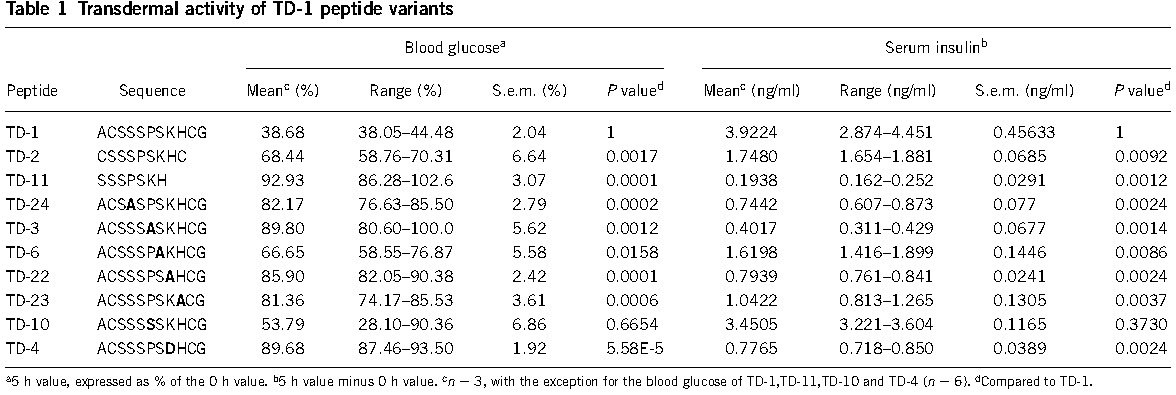
The transdermal-enhancing activity of the peptide was sequence specific(figure 2) and dose dependent, did not involve direct interaction with target proteins(figure 3). | The transdermal-enhancing activity of the peptide was sequence specific(figure 2) and dose dependent, did not involve direct interaction with target proteins(figure 3). | ||
| + | |||
[[Image:USTC_China_iGEM13_BBa_K1074000_2.png|thumb|center|900px|figure 2 Transdermal activity of TD-1 peptide variants]] | [[Image:USTC_China_iGEM13_BBa_K1074000_2.png|thumb|center|900px|figure 2 Transdermal activity of TD-1 peptide variants]] | ||
[[Image:USTC_China_iGEM13_BBa_K1074000_3.png|thumb|center|900px|figure 3 Exploring TD-1’s mode of action. (a) TD-1 does not bind insulin directly. 125I-insulin was added to ELISA microwell plates precoated with | [[Image:USTC_China_iGEM13_BBa_K1074000_3.png|thumb|center|900px|figure 3 Exploring TD-1’s mode of action. (a) TD-1 does not bind insulin directly. 125I-insulin was added to ELISA microwell plates precoated with | ||
| Line 20: | Line 21: | ||
to the same skin site. Serum insulin (c) and blood glucose (d) levels were measured before TD-1 treatment and 5 h after insulin administration. | to the same skin site. Serum insulin (c) and blood glucose (d) levels were measured before TD-1 treatment and 5 h after insulin administration. | ||
Coadministration (CO) of TD-1 (500 mg) and insulin (70 mg) served as the control. Mean ± s.e.m., (n ¼ 6 for glucose and n Z 3 for insulin).]] | Coadministration (CO) of TD-1 (500 mg) and insulin (70 mg) served as the control. Mean ± s.e.m., (n ¼ 6 for glucose and n Z 3 for insulin).]] | ||
| + | |||
| + | When you fuse TD1 with your target proteins, N-terminal modified and a linker of GGGS are recommended. | ||
===References=== | ===References=== | ||
Revision as of 03:01, 27 September 2013
TD1, Transdermal peptide
TD1 is a short synthetic peptide(ACSSSPSKHCG)identified by in vivo phage display, facilitated efficient transdermal protein delivery through intact skin. Studies suggested that the peptide creates a transient opening in the skin barrier to enable macromolecular material to reach systemic circulation.
Usage and Biology
TD1 can facilitated transdermal protein delivery by Coadministration of it with target protein or fused with the target protein(always N-terminal).Studies shows that Coadministration of the peptide and insulin to the abdominal skin of diabetic rats resulted in elevated systemic levels of insulin and suppressed serum glucose levels for at least 11 h. Significant systemic bioavailability of human growth hormone was also achieved when topically coadministered with the peptide(figure 1).

The transdermal-enhancing activity of the peptide was sequence specific(figure 2) and dose dependent, did not involve direct interaction with target proteins(figure 3).

When you fuse TD1 with your target proteins, N-terminal modified and a linker of GGGS are recommended.
References
Chen, Y.P., et al., Transdermal protein delivery by a coadministered peptide identified via phage display. Nature biotechnology, 2006. 24(4): p. 455-460.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]