Difference between revisions of "Part:BBa K1159001"
m (→Usage and Biology) |
|||
| Line 6: | Line 6: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | |||
| + | |||
| + | ===Experimental Data=== | ||
| + | ===Production in ''E. coli'' and purification=== | ||
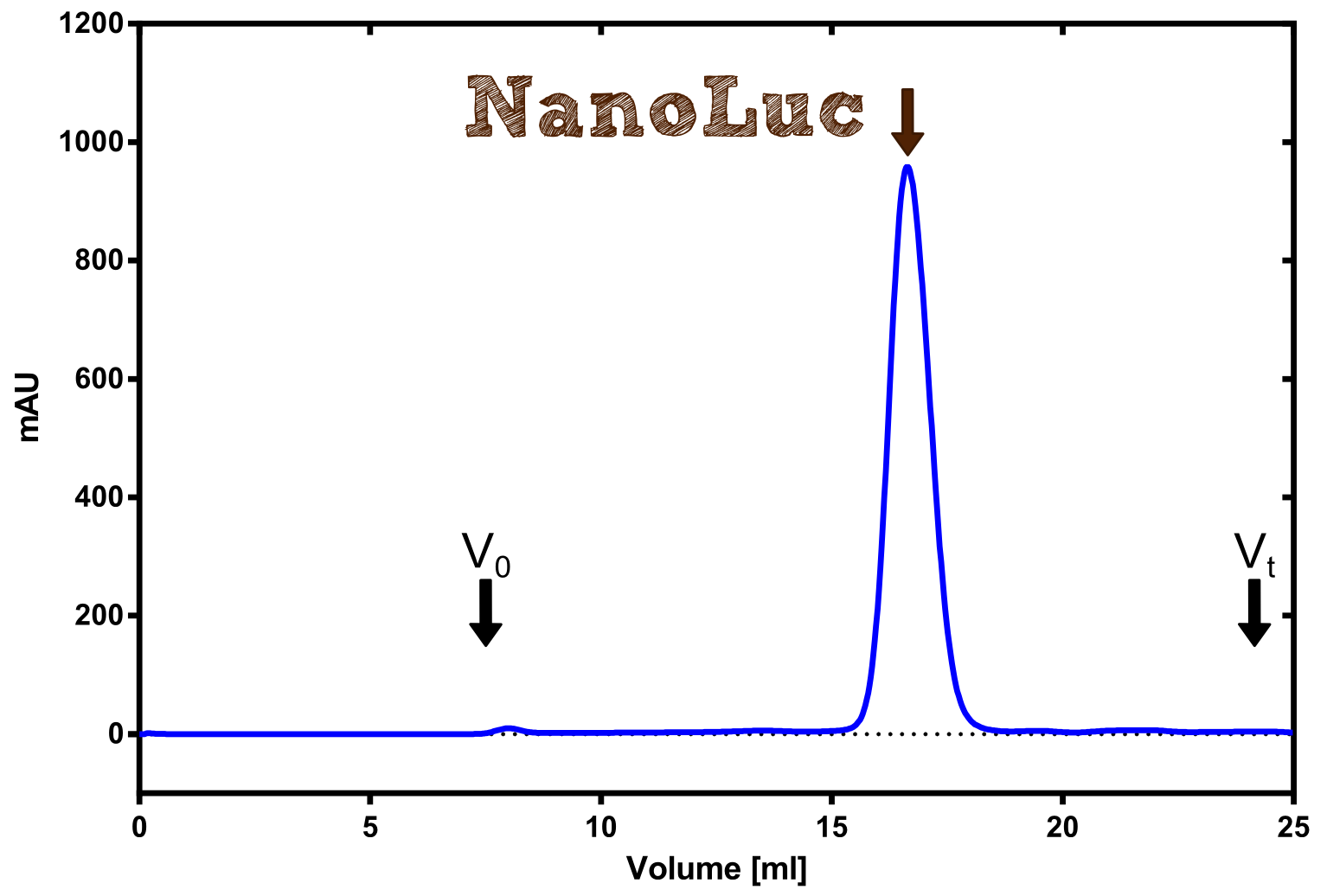
| + | [[File:TUM13_Analytprep_NanoLuc.png|thumb|right|320px| '''Figure 19:''' Analytical size exclusion chromatography on a Superdex 200 10/30 column showing a single elution peak for the NanoLuc.]] | ||
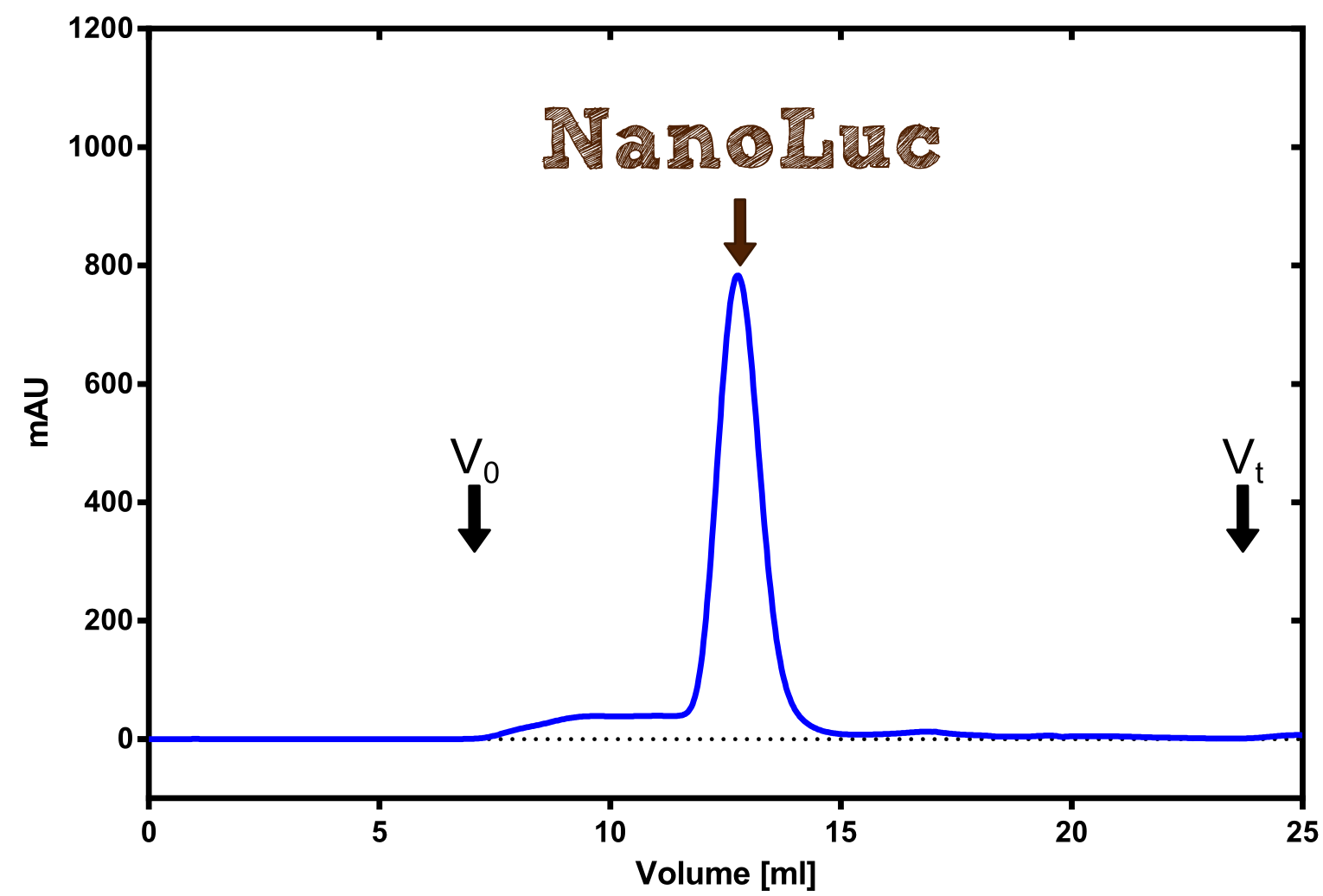
| + | [[File:TUM13_Preparative_NanoLuc.png|thumb|right|320px| '''Figure 20a:''' Preperative size exclusion chromatography on a Superdex 75 10/30 column showing a single elution peak for the NanoLuc.]] | ||
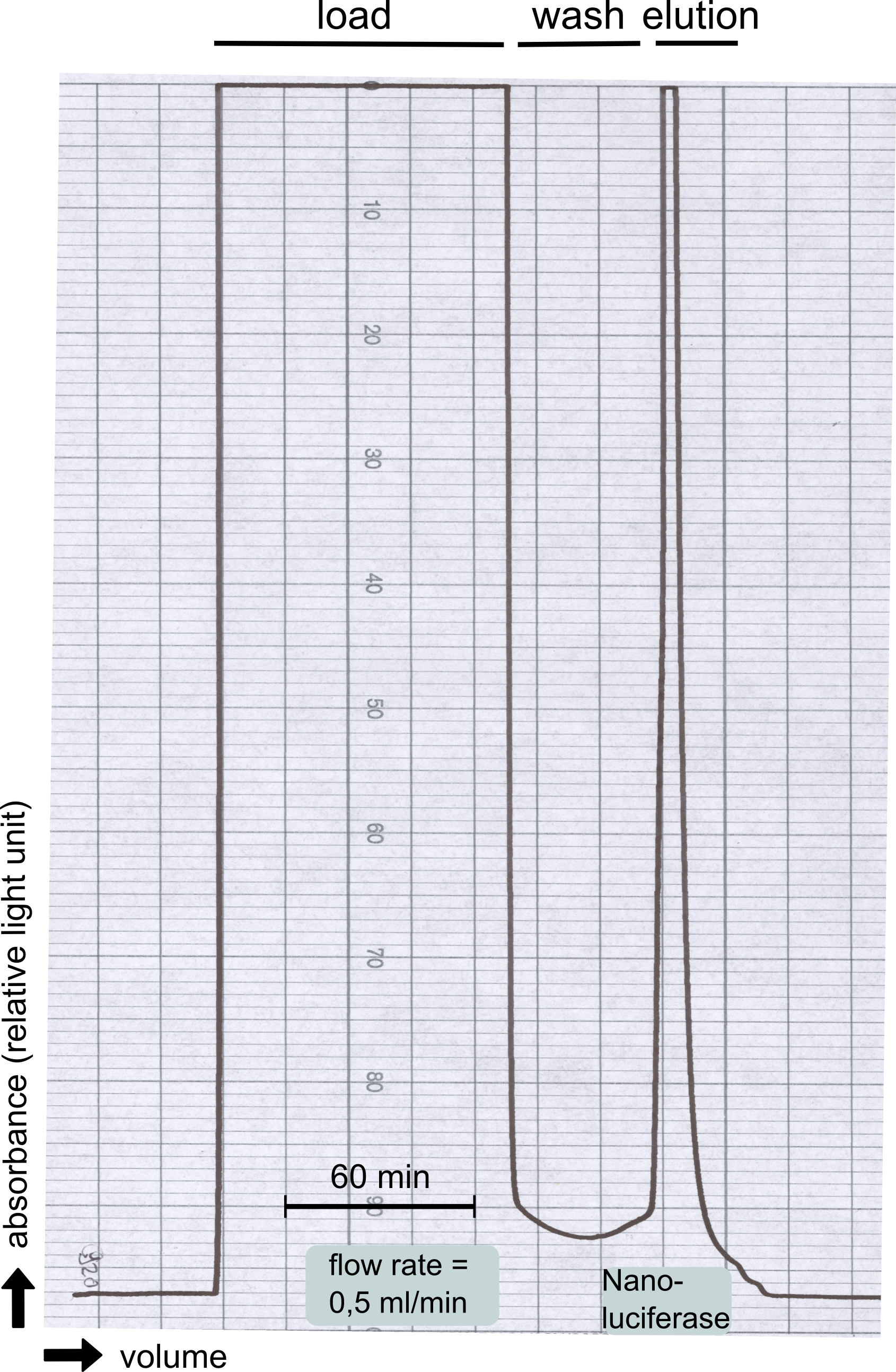
| + | [[File:TUM13_Nanoluciferase_chromatogramm.png|thumb|left|320px| '''Figure 18: '''Streptavidin affinity chromatography for NanoLuc]] | ||
| + | Therefore the NanoLuc was synthesized as a BioBrick in RFC[25] and was produced in ''E. coli'' using the pBad expression system with a C-terminal ''Strep''-tag. After the production (2 l of LB-media for analytical and 12 l for preparative preparations) the cells were disrupted using sonification and the lysate was dialysed against 5 l of 1x SA-buffer. Afterwards the lysate was applied to a Streptavidin-Affinity (SA) column and was subsequently washed using SA-Buffer until a baseline was reached and the protein was then eluted using 5 mM of biotin (Attention: These are special columns which are not availible commercially. If you are using commercial colum material you have to use d-Desthiobiotin because usual biotin will elute your protein but you will not be able to regenerate the column after your chromatography). After the SA-chromatography the protein was concentrated using centrifugal concentration units (MWCO: 10 kDa). The concentrated protein was then applied on a Superdex S200/75 size exclusion chromatography. The chromatogram of both preparations show a single peak in the chromatogram which elutes at an expected elution volume of 15 ml. The absence of any notable aggregation peak shows the high stability of this protein and the ease of production. | ||
| + | |||
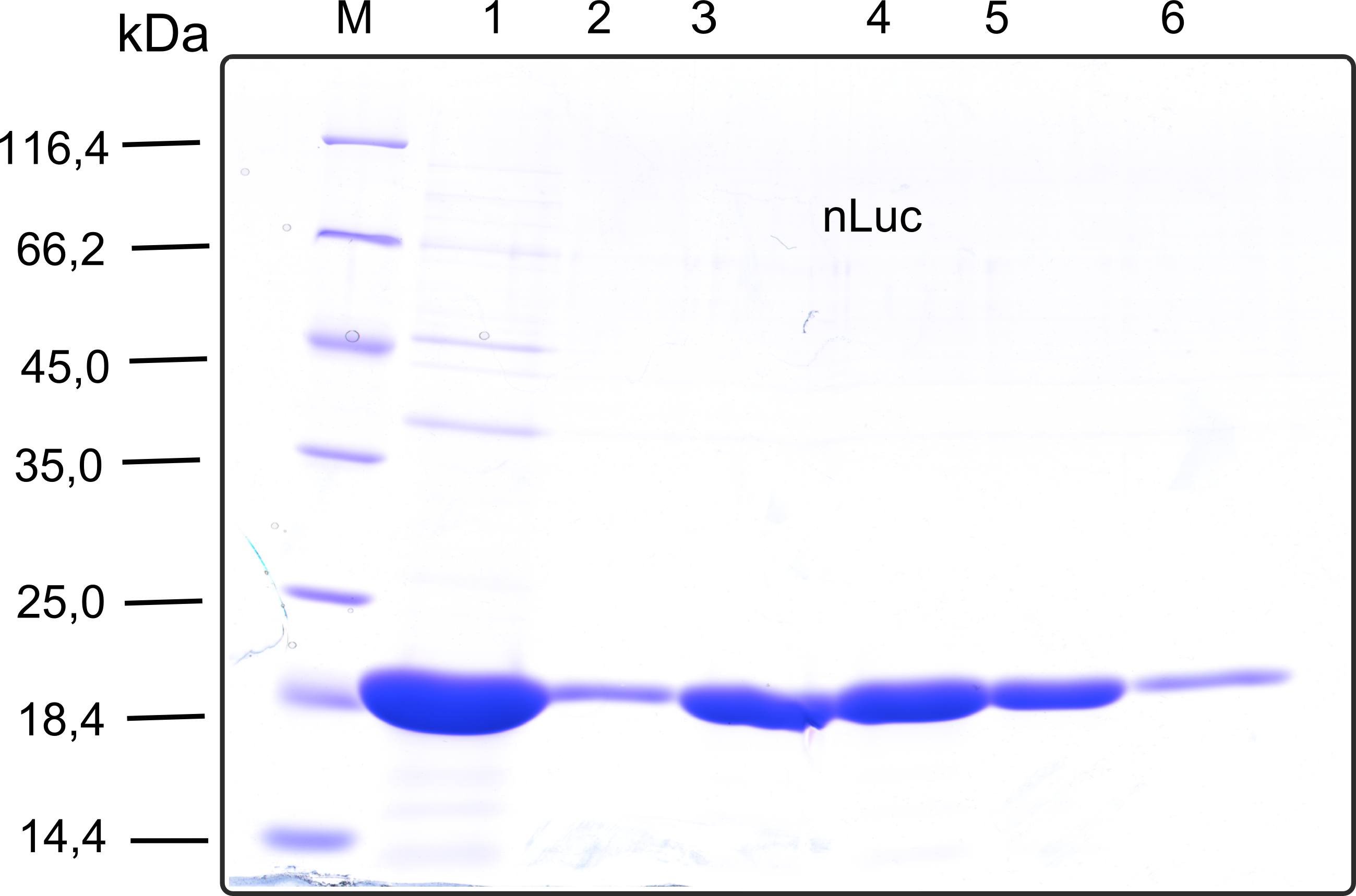
| + | [[File:TUM13_SDS_nLuc.png|thumb|right|320px|'''Figure 20b:'''SDS-gel of recombinant nLuc with the marker (M) followed by the concentrated throughput of the streptavidin affinity column and 6 fractions collected from the elution peak]] | ||
| + | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
Revision as of 03:48, 5 October 2013
NanoLuc Luciferase in RFC[25]
NanoLuc Luciferase is engineered ATP-independent luciferase from a deep-sea shrimp which luminescense 2 magnitues higher than these from Renilla reniformis or from Photinus pyralis (firefly). Also the molecular weight of NanoLuc luciferase is twice smaller compared to other luciferase (only 19 kDa).
Usage and Biology
Experimental Data
Production in E. coli and purification
Therefore the NanoLuc was synthesized as a BioBrick in RFC[25] and was produced in E. coli using the pBad expression system with a C-terminal Strep-tag. After the production (2 l of LB-media for analytical and 12 l for preparative preparations) the cells were disrupted using sonification and the lysate was dialysed against 5 l of 1x SA-buffer. Afterwards the lysate was applied to a Streptavidin-Affinity (SA) column and was subsequently washed using SA-Buffer until a baseline was reached and the protein was then eluted using 5 mM of biotin (Attention: These are special columns which are not availible commercially. If you are using commercial colum material you have to use d-Desthiobiotin because usual biotin will elute your protein but you will not be able to regenerate the column after your chromatography). After the SA-chromatography the protein was concentrated using centrifugal concentration units (MWCO: 10 kDa). The concentrated protein was then applied on a Superdex S200/75 size exclusion chromatography. The chromatogram of both preparations show a single peak in the chromatogram which elutes at an expected elution volume of 15 ml. The absence of any notable aggregation peak shows the high stability of this protein and the ease of production.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 10
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]