Difference between revisions of "Part:BBa K863021"
KevinJarosch (Talk | contribs) (Undo revision 160876 by KevinJarosch (Talk)) |
KevinJarosch (Talk | contribs) (Undo revision 160878 by KevinJarosch (Talk)) |
||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
| − | <partinfo> | + | <partinfo>BBa_K863021 short</partinfo> |
| − | bhal laccase | + | bhal laccase from Bacillus halodurans |
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 9: | Line 9: | ||
<!-- --> | <!-- --> | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
| − | <partinfo> | + | <partinfo>BBa_K863021 SequenceAndFeatures</partinfo> |
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
===Functional Parameters=== | ===Functional Parameters=== | ||
| − | <partinfo> | + | <partinfo>BBa_K863021 parameters</partinfo> |
<!-- --> | <!-- --> | ||
| − | + | ==Activity Analysis of [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL]== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | == | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | = | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<p align="justify"> | <p align="justify"> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
===Initial activity tests of purified fractions=== | ===Initial activity tests of purified fractions=== | ||
| − | The resulting fractions of the cultivation and purification of [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] (fraction 1 to 5) were analysed with activity tests. After rebuffering into deionized H<sub>2</sub>O and incubation with 0.4 mM CuCl<sub>2</sub> for 2 hours, the samples were measured with 140 µL sample, 0.1 mM ABTS, 100 mM sodium acetate buffer to a final volume of 200 µL. The change in optical density was measured at 420 nm, reporting the oxidation of ABTS for 5 hours at 25°C. An increase in ABTS<sub>ox</sub> can be seen (Figure | + | The resulting fractions of the cultivation and purification of [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] (fraction 1 to 5) were analysed with activity tests. After rebuffering into deionized H<sub>2</sub>O and incubation with 0.4 mM CuCl<sub>2</sub> for 2 hours, the samples were measured with 140 µL sample, 0.1 mM ABTS, 100 mM sodium acetate buffer to a final volume of 200 µL. The change in optical density was measured at 420 nm, reporting the oxidation of ABTS for 5 hours at 25°C. An increase in ABTS<sub>ox</sub> can be seen (Figure 4), indicating produced [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] laccase in each fraction. Fraction 2 shows the highest amount of ABTS<sub>ox</sub> (55%) reaching saturation after 3 hours. Similar to [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863000 BPUL] laccase, [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] is capable to reach saturation after 3 hours with approximately oxidizing 55% of the supplied ABTS. Therefore [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] is going to be characterized further. |
| − | [[Image:Bielefeld2012_17_09_BHAL1.jpg|thumbnail|center|500px|'''Figure | + | [[Image:Bielefeld2012_17_09_BHAL1.jpg|thumbnail|center|500px|'''Figure 4''': Activity test of [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] fractions after purification. Reaction setup includes 140 µL fraction sample (CuCl2 incubated), 0.1 mM ABTS and 100 mM sodium actetate buffer (pH 5) to a final volume of 200 µL. Measurements were done at 25°C and over a time period of 5 hours. Each fraction shows activity, especially fraction 2, which therefore contains most [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] laccase. (n=4)]] |
| Line 92: | Line 28: | ||
===Initial activity tests of purified fractions=== | ===Initial activity tests of purified fractions=== | ||
| − | Different fractions of the purification of a new cultivation since the Regional Jamborees in Amsterdam were tested regarding their activity of the produced BHAL. Before and after re-buffering the protein concentration was determined. The initial activity tests were done in Britton-Robinson buffer (pH 5) with 0.1 mM ABTS at 25 °C. The protein amount was adjusted in each sample for a comparison. One distinct fraction showed the highest activity: fraction 5% 3 (Fig. | + | Different fractions of the purification of a new cultivation since the Regional Jamborees in Amsterdam were tested regarding their activity of the produced BHAL. Before and after re-buffering the protein concentration was determined. The initial activity tests were done in Britton-Robinson buffer (pH 5) with 0.1 mM ABTS at 25 °C. The protein amount was adjusted in each sample for a comparison. One distinct fraction showed the highest activity: fraction 5% 3 (Fig. 5). The contained laccase amount was calculated by assuming that the most active fraction contains 90 % laccase. This leads to a BHAL concentration of 10,9 ng mL<sup>-1</sup>. |
| − | [[Image:Bielefeld2012_new_BHAL_activity.jpg|500px|thumb|center|'''Figure | + | [[Image:Bielefeld2012_new_BHAL_activity.jpg|500px|thumb|center|'''Figure 5:''' Activity assay of each purified fraction of recent produced BHAL. Samples were re-buffered into H<sub>2</sub>O and the protein amount in each fraction had been adjusted. The measurements were done using the [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Analytics#General_setup_of_enzyme_activity_measurements/ standard activity assay protocol] over night. The first number indicates the percentage of used elution buffer, whereas the second number stands for the fraction number of this elution.]] |
===[https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] activity depending on different ABTS concentrations=== | ===[https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] activity depending on different ABTS concentrations=== | ||
| − | To be able to calculate the activity in Units mg<sup>-1</sup>, measurements had to be done under substrate saturation. This allows the comparison of Units mg<sup>-1</sup> with other laccase activities and data found in literature. For this purpose ABTS concentrations ranging from 0.1 mM to 8 mM were applied in an experimental setup containing Britton-Robinson buffer (pH) and a temperature of 25 °C. For measurements with 0.1 mM to 5 mM ABTS 616 ng BHAL were used (Fig. | + | To be able to calculate the activity in Units mg<sup>-1</sup>, measurements had to be done under substrate saturation. This allows the comparison of Units mg<sup>-1</sup> with other laccase activities and data found in literature. For this purpose ABTS concentrations ranging from 0.1 mM to 8 mM were applied in an experimental setup containing Britton-Robinson buffer (pH) and a temperature of 25 °C. For measurements with 0.1 mM to 5 mM ABTS 616 ng BHAL were used (Fig. 6). For measurements with 5 mM to 8 mM ABTS only 308 ng BHAL were applied (Fig. 7). Applying less than 7 mM ABTS a static increase in oxidized ABTS was given. Measurements with 8 mM ABTS showed a slower increase in oxidized ABTS as with 7 mM ABTS (Fig. 7). This may be due to a substrate toxication. The most compromising ABTS concentration was 7 mM with the highest increase in oxidized ABTS. Therefore a substrate saturation was reached with 7 mM ABTS. |
| − | [[Image:Bielefeld2012_BHAL_klein_ABTS.jpg|thumb|left|360px|'''Figure | + | [[Image:Bielefeld2012_BHAL_klein_ABTS.jpg|thumb|left|360px|'''Figure 6:''' Activity assay to determine the substrate saturation with ABTS as a substrate. Measurements were done with 616 ng BHAL laccase in Britton-Robinson buffer (pH 5) at 25 °C. ABTS concentrations ranged from 0.1 mM to 5 mM.]] |
| − | [[Image:Bielefeld2012_BHAL_ABTS_hoch.jpg|thumb|right|360px|'''Figure | + | [[Image:Bielefeld2012_BHAL_ABTS_hoch.jpg|thumb|right|360px|'''Figure 7:''' Activity assay to determine the substrate saturation with ABTS as a substrate. Measurements were done with 308 ng BHAL laccase in Britton-Robinson buffer (pH 5) at 25 °C. ABTS concentrations ranged from 5 mM to 8 mM. An ABTS concentration of 7 mM was determined as substrate saturated.]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
| Line 105: | Line 41: | ||
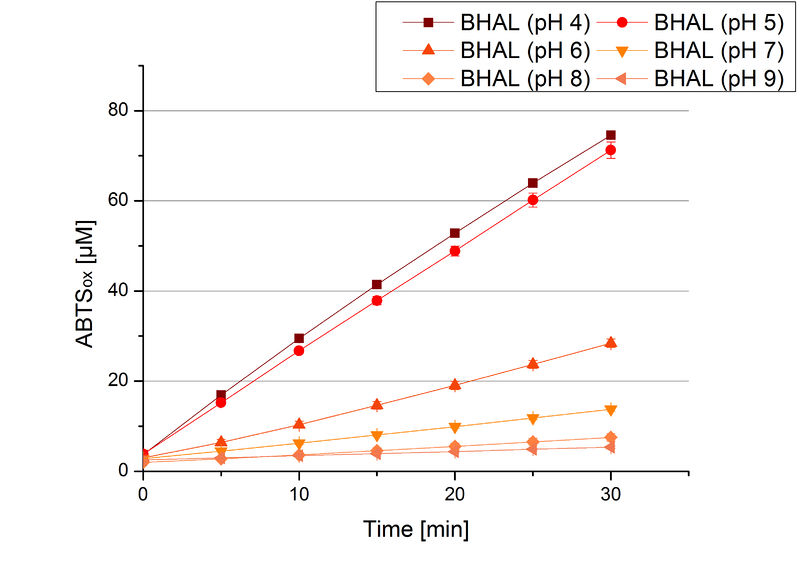
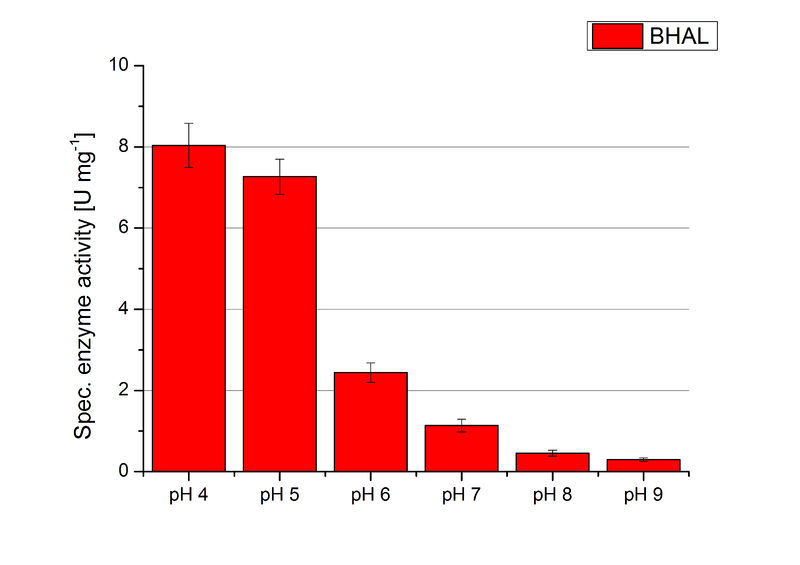
=== [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] pH optimum=== | === [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] pH optimum=== | ||
| − | [[Image:Bielefeld2012_Halo_pH_Foto.png|thumb|right|200px|'''Figure | + | [[Image:Bielefeld2012_Halo_pH_Foto.png|thumb|right|200px|'''Figure 8:''' Microtiter plate of the measurements for pH optimum determination. The more intensive the blue color, the more ABTS got oxidized. At pH 4 and pH 5 the darkest colour was detected.]] |
| − | To determine the optimal experimental setup for BHAL activity measurements, the best pH had to be determined. Using Britton-Robinson buffer pHs between pH 4 and pH 9 had been adjusted. 308 ng BHAL per well had been tested under these pH conditions using 7 mM ABTS. The CuCl<sub>2</sub> incubated and therefor activated BHAL showed a high activity at pH 4 and pH 5, where most of ABTS was oxidized (compared to Fig. | + | To determine the optimal experimental setup for BHAL activity measurements, the best pH had to be determined. Using Britton-Robinson buffer pHs between pH 4 and pH 9 had been adjusted. 308 ng BHAL per well had been tested under these pH conditions using 7 mM ABTS. The CuCl<sub>2</sub> incubated and therefor activated BHAL showed a high activity at pH 4 and pH 5, where most of ABTS was oxidized (compared to Fig. 8 and 9). The calculated specific enzyme activity of BHAL showed high activity at both mentioned pHs (Fig. 10). While BHAL had an activity of ~8 U mg<sup>-1</sup> at pH 4 and pH 5, the enzyme activity decreased at higher pHs. At a pH of 6 only 1/3 of enzyme activity could be detected compared to the activity at pH 4 and pH 5. While still active at pH 7, the BHAL is not as suitable as thought for an application at a waste water treatment plant because of its high activity in acidic environments. |
<br style="clear: both" /> | <br style="clear: both" /> | ||
| − | [[Image:Bielefeld2012_BHAL_pH_new.jpg|thumb|360px|left|'''Figure | + | [[Image:Bielefeld2012_BHAL_pH_new.jpg|thumb|360px|left|'''Figure 9:''' Oxidized ABTS by BHAL at different pH adjustments. The experimental setup included CuCl<sub>2</sub> incubated BHAL (308 ng), Britton Robinson buffer adjusted to the tested pHs and 5 mM ABTS. Measurements were done at 25 °C for 30 minutes. The highest amount of oxidzed ABTS could be detected at pH 4 and pH 5.]] |
| − | [[Image:Bielefeld2012_BHAL_pH_Units.jpg|thumb|360px|right|'''Figure | + | [[Image:Bielefeld2012_BHAL_pH_Units.jpg|thumb|360px|right|'''Figure 10:''' Calculated specific enzyme activity of BHAL at different pH conditions. The highest specific enzyme activity for ABTS was under pH 4 and pH 5 conditions. The higher the pH, the less ABTS got oxidzed. One unit is defined as the amount of laccase that oxidizes 1 μmol of ABTS substrate per minute.]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
=== [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] activity at different temperatures=== | === [https://parts.igem.org/wiki/index.php?title=Part:BBa_K863022 BHAL] activity at different temperatures=== | ||
| − | [[Image:Bielefeld2012 BHAL Temp ABTSox.jpg|left|200px|thumb|'''Figure | + | [[Image:Bielefeld2012 BHAL Temp ABTSox.jpg|left|200px|thumb|'''Figure 11:''' Standard activity test for BHAL measured at 10 °C and 25 °C resulting in a decreased activity at 10 °C. As a negative control the impact of 0.4 mM CuCl<sub>2</sub> in oxidizing ABTS at 10 °C and 25 °C was analyzed.]] |
| − | [[Image:Bielefeld2012 BHAL Temp Units.jpg|right|200px|thumb|'''Figure | + | [[Image:Bielefeld2012 BHAL Temp Units.jpg|right|200px|thumb|'''Figure 12:''' Deriving from the obtained values of oxidized ABTS in time at 10 °C and 25 °C the specific enzyme activity was calculated. For the temperatures a difference of 3 U mg<sup>-1</sup> could be detected. One unit is defined as the amount of laccase that oxidizes 1 μmol of ABTS substrate per minute.]] |
| − | To investigate the activity of BHAL at temperatures that will apply at a waste water treatment plant throughout the year, activity tests were performed at 10 °C and 25 °C as described above. The measurements were conducted for 30 minutes. The obtained results revealed a lower activity of BHAL at 10 °C in comparison to 25 °C (see Fig. | + | To investigate the activity of BHAL at temperatures that will apply at a waste water treatment plant throughout the year, activity tests were performed at 10 °C and 25 °C as described above. The measurements were conducted for 30 minutes. The obtained results revealed a lower activity of BHAL at 10 °C in comparison to 25 °C (see Fig. 11). The obtained results were used to calculate the specific enzyme activity which was at 4.2 and 7.2 U mg<sup>-1</sup>, respectively (see Figure 12). The negative control without BHAL but 0.4 mM CuCl<sub>2</sub> at 10 °C and 25 °C showed a negligible oxidation of ABTS. The activity of BHAL was increased to about 60 % at 10 °C but nevertheless the observed activity at both conditions was great news for the possible application in waste water treatment plants. |
<br><br><br><br> | <br><br><br><br> | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
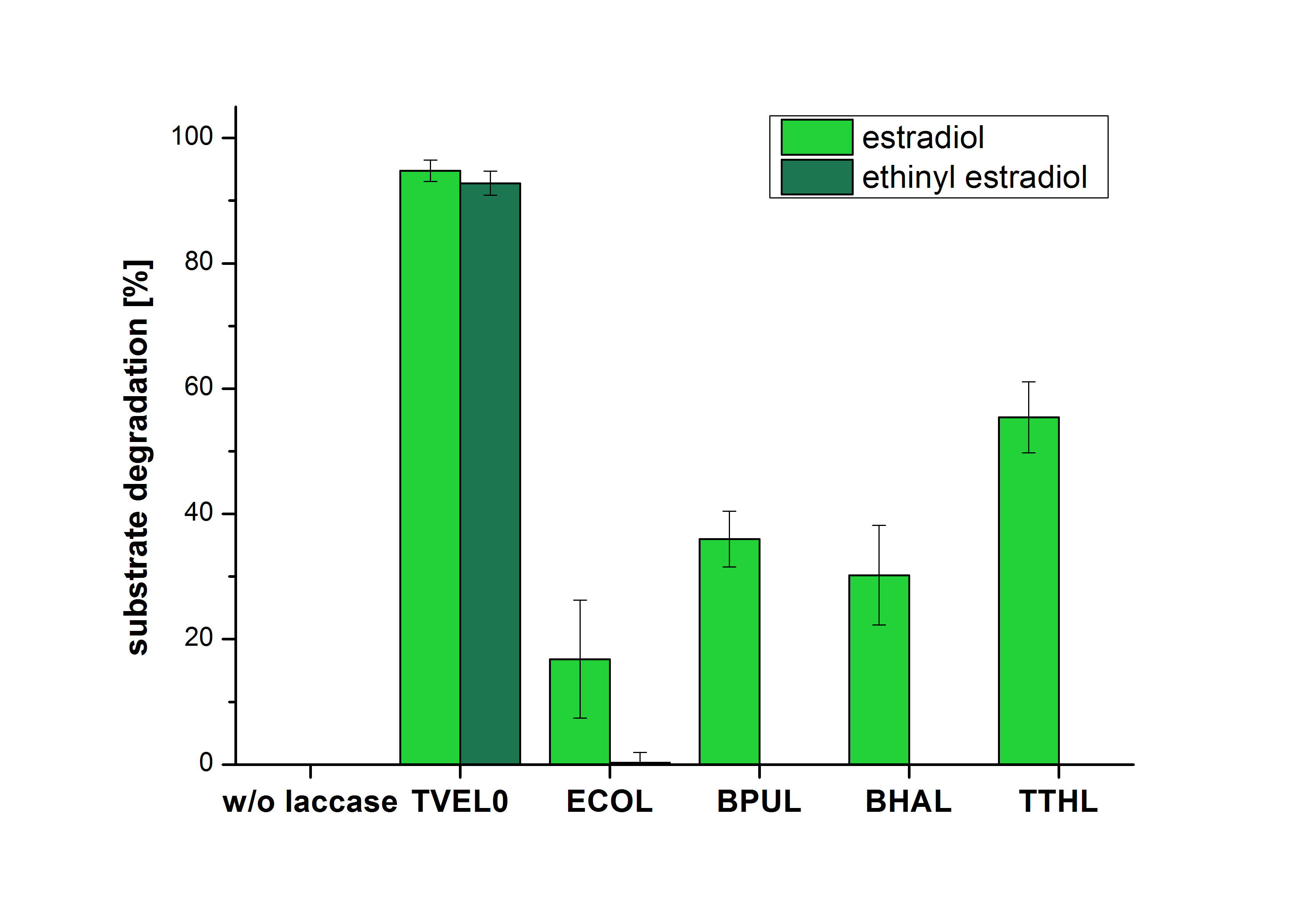
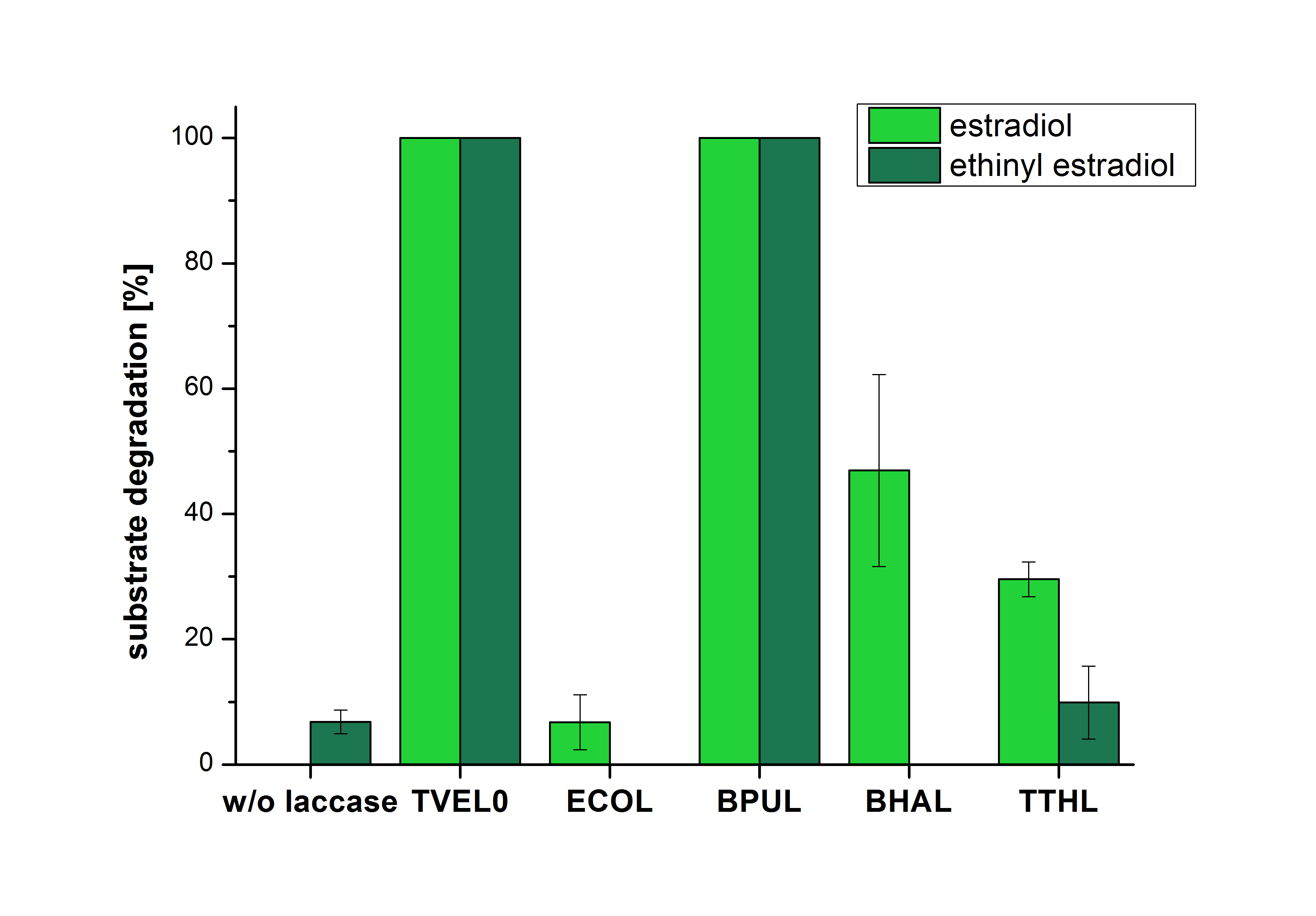
| Line 123: | Line 59: | ||
| − | The results of the reactions of the laccases with addition of ABTS are shown in Figure | + | The results of the reactions of the laccases with addition of ABTS are shown in Figure 3. The experimental set ups were the same as the reaction approach without ABTS described above. The X-axis displays the different tested laccases. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are shown. The degradation was measured at t<sub>0</sub> and after five hours of incubation at 20 °C. The negative control showed no degradation of estradiol. 6.8 % of ethinyl estradiol was decayed. The positive control TVEL0 is able to degrade 100 % estradiol and ethinyl estradiol. The laccase BPUL (from ''Bacillus pumilus'') degraded 46.9 % of used estradiol after ten minutes incubation. ECOL was able to degrade 6.7 % estradiol. BHAL degraded 46.9 % estradiol. With TTHL (laccase from ''Thermus thermophilus'')a degradation 29.5 % were determined. |
| − | [[Image:Bielefeld2012_Ohne_ABTS.jpg|400px|thumb|left|'''Figure | + | [[Image:Bielefeld2012_Ohne_ABTS.jpg|400px|thumb|left|'''Figure 2: Degradation of estradiol (dark green) and ethinyl estradiol (light green) with the different laccases after 5 hours without ABTS.''' In the graph it is shown that the bought laccase TVEL0 which was used as positive control is able to degrade more than 90 percent of the used substrates. None of the bacterial laccases are able to degrade ethinyl estradiol without ABTS but estradiol is degraded in a range from 16 %(ECOL) to 55 % (TTHL). The original concentrations of substrates were 2 µg per approach. (n = 4)]] |
| − | [[Image:Bielefeld2012_Mit_ABTS.jpg|400px|thumb|right|'''Figure | + | [[Image:Bielefeld2012_Mit_ABTS.jpg|400px|thumb|right|'''Figure 3: Degradation of estradiol (blue) and ethinyl estradiol (red) with the different laccases after 10 minutes hours with ABTS added.''' The commercial laccase TVEL0 which was used as positive control is able to degrade all of the used substrates. The bacterial laccase BPUL degraded 100 % of ethinyl estradiol and estradiol. ECOL the laccase from ''E. coli'' degraded 6.7 % estradiol and none of the used ethinyl estradiol. BHAL degraded 46.9 % of estradiol but no ethinyl estradiol. The laccase TTHL from ''Thermus thermophilus'' degraded 29.5 % of estradiol and 9.8 % ethinyl estradiol. The original concentrations of substrates were 2 µg per approach. (n = 4)]] |
<br style="clear: both" /> | <br style="clear: both" /> | ||
| Line 131: | Line 67: | ||
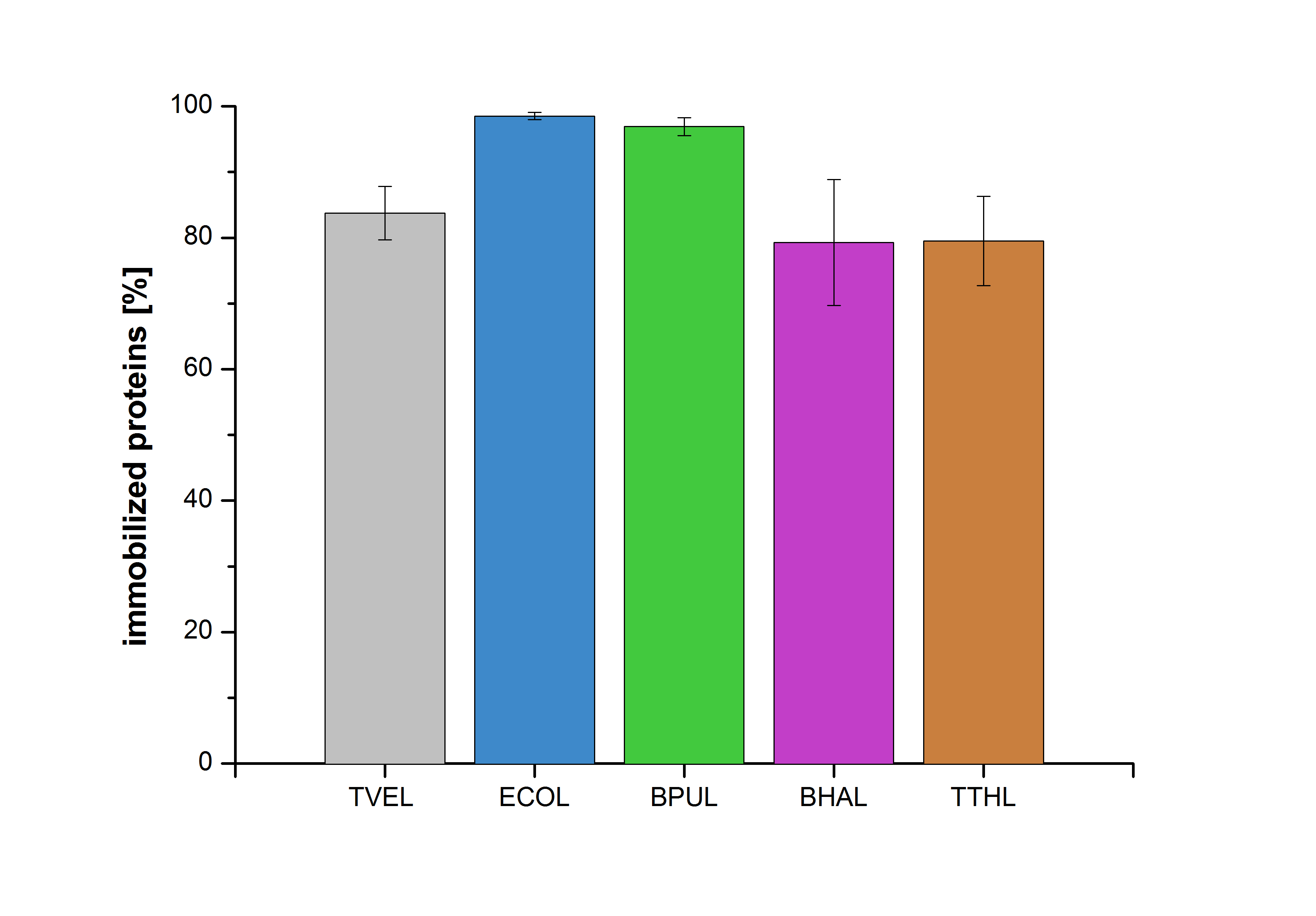
| − | [[Image:Bielefeld2012-Immobilized_proteins.jpg|500px|left|thumb|'''Figure | + | [[Image:Bielefeld2012-Immobilized_proteins.jpg|500px|left|thumb|'''Figure 20''': The percentage of laccases immobilized to CPC-Beads. 99 % of ECOL, 97 % of BPUL and 79 % of BHAL and TTHL laccases were bound to the beads.]] |
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
| − | Figure | + | Figure 20 shows the percentage of laccases bound after incubation with CPC-beads, relative to the original concentration. The concentration of laccases in the supernatant after incubation was measured using Roti®-Nanoquant. The results showed that only 21% of BHAL laccases was still present in the supernatant. This illustrates that BHAL was successfully immobilized on the CPC-beads. |
</div> | </div> | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
| Line 140: | Line 76: | ||
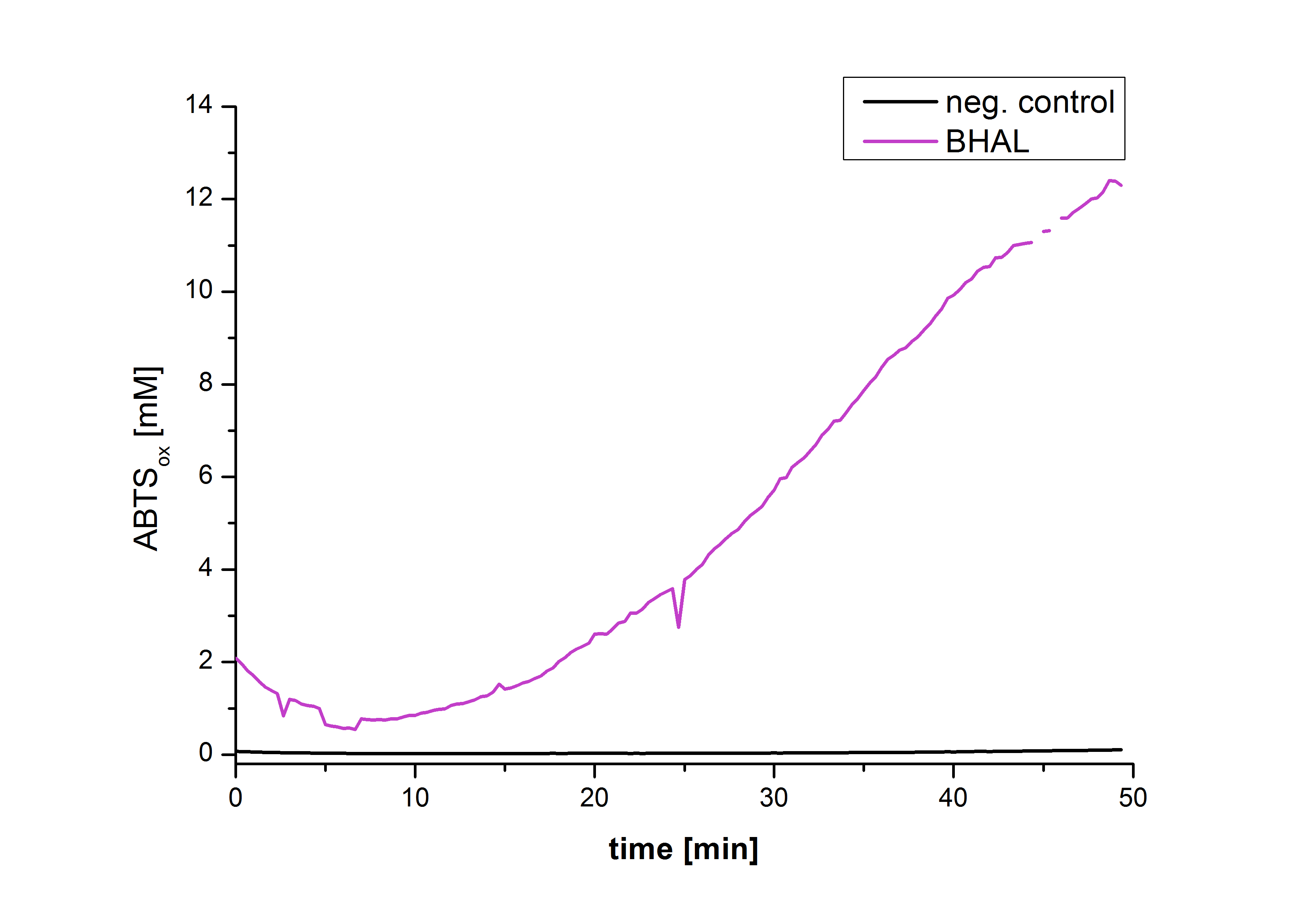
| − | [[Image:Bielefeld2012-Graphen_Bead_Halo.jpg|500px|left|thumb|'''Figure | + | [[Image:Bielefeld2012-Graphen_Bead_Halo.jpg|500px|left|thumb|'''Figure 22''': Illustration of ABTS oxidation by BHAL with time compared to the negative control. The increase in ABTS oxidized proves laccase activity.]] |
<div style="text-align:justify;"> | <div style="text-align:justify;"> | ||
| − | Figure | + | Figure 22 shows the illustration of ABTS oxidation by BHAL with time compared to the negative control. The increase in ABTS oxidized proves laccase activity even if a direct comparison with the original and not immobilized laccase solution was not possible due to the very low concentration of purified BHAL. |
<br style="clear: both" /> | <br style="clear: both" /> | ||
Revision as of 03:47, 27 October 2012
bhal laccase from Bacillus halodurans
bhal laccase from Bacillus halodurans
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 157
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Activity Analysis of BHAL
Initial activity tests of purified fractions
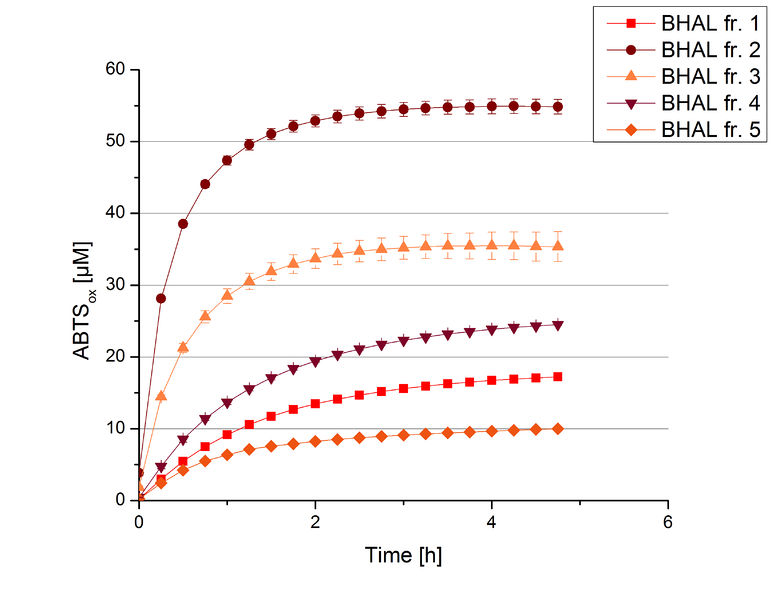
The resulting fractions of the cultivation and purification of BHAL (fraction 1 to 5) were analysed with activity tests. After rebuffering into deionized H2O and incubation with 0.4 mM CuCl2 for 2 hours, the samples were measured with 140 µL sample, 0.1 mM ABTS, 100 mM sodium acetate buffer to a final volume of 200 µL. The change in optical density was measured at 420 nm, reporting the oxidation of ABTS for 5 hours at 25°C. An increase in ABTSox can be seen (Figure 4), indicating produced BHAL laccase in each fraction. Fraction 2 shows the highest amount of ABTSox (55%) reaching saturation after 3 hours. Similar to BPUL laccase, BHAL is capable to reach saturation after 3 hours with approximately oxidizing 55% of the supplied ABTS. Therefore BHAL is going to be characterized further.
Initial activity tests of purified fractions
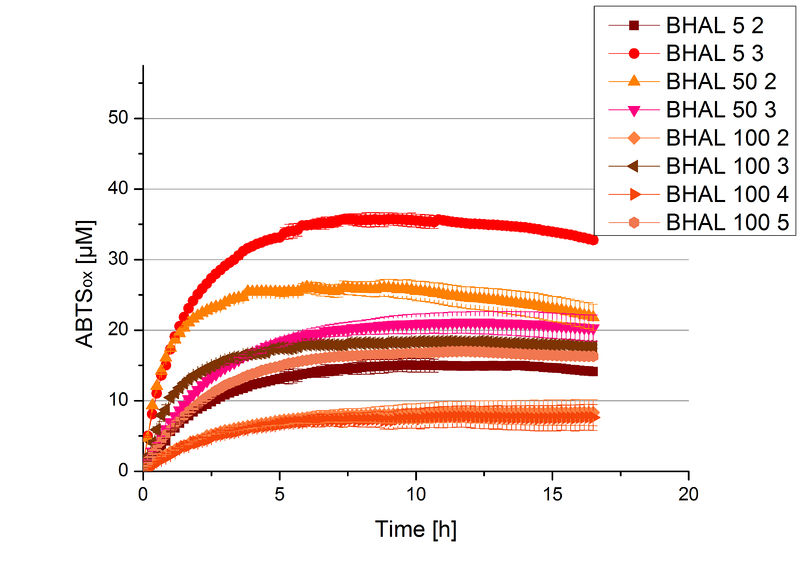
Different fractions of the purification of a new cultivation since the Regional Jamborees in Amsterdam were tested regarding their activity of the produced BHAL. Before and after re-buffering the protein concentration was determined. The initial activity tests were done in Britton-Robinson buffer (pH 5) with 0.1 mM ABTS at 25 °C. The protein amount was adjusted in each sample for a comparison. One distinct fraction showed the highest activity: fraction 5% 3 (Fig. 5). The contained laccase amount was calculated by assuming that the most active fraction contains 90 % laccase. This leads to a BHAL concentration of 10,9 ng mL-1.
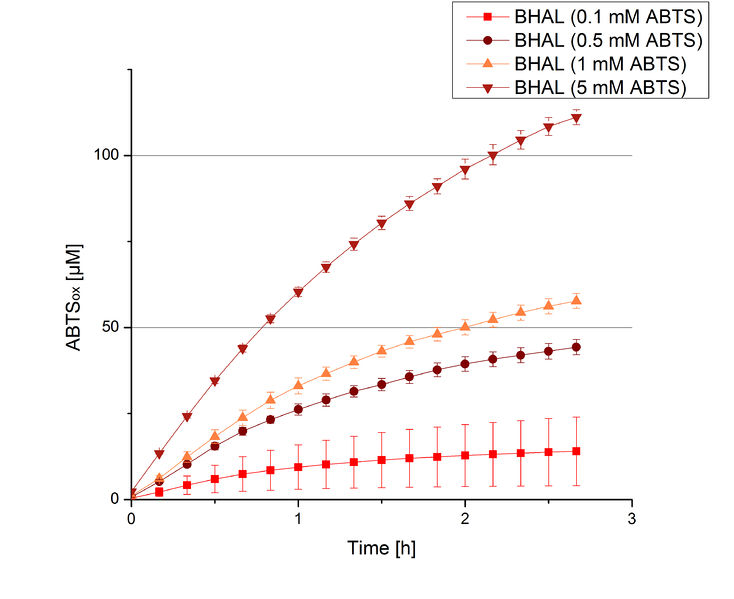
BHAL activity depending on different ABTS concentrations
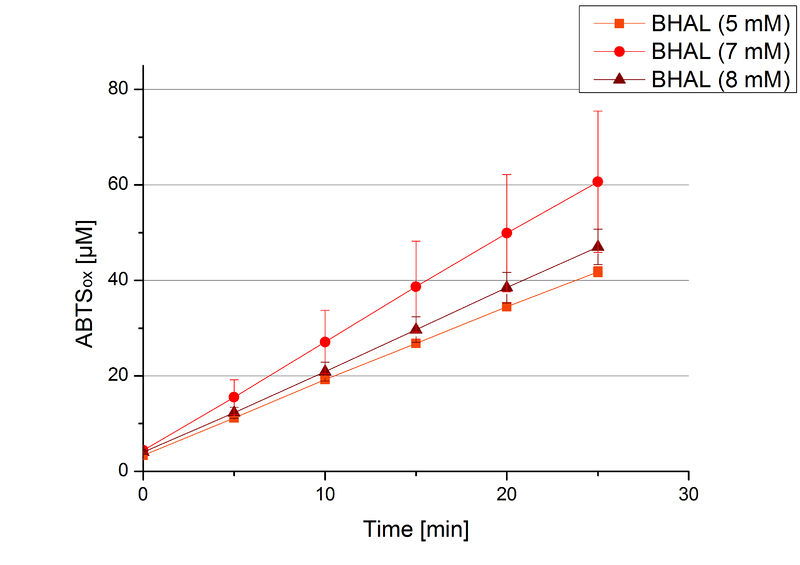
To be able to calculate the activity in Units mg-1, measurements had to be done under substrate saturation. This allows the comparison of Units mg-1 with other laccase activities and data found in literature. For this purpose ABTS concentrations ranging from 0.1 mM to 8 mM were applied in an experimental setup containing Britton-Robinson buffer (pH) and a temperature of 25 °C. For measurements with 0.1 mM to 5 mM ABTS 616 ng BHAL were used (Fig. 6). For measurements with 5 mM to 8 mM ABTS only 308 ng BHAL were applied (Fig. 7). Applying less than 7 mM ABTS a static increase in oxidized ABTS was given. Measurements with 8 mM ABTS showed a slower increase in oxidized ABTS as with 7 mM ABTS (Fig. 7). This may be due to a substrate toxication. The most compromising ABTS concentration was 7 mM with the highest increase in oxidized ABTS. Therefore a substrate saturation was reached with 7 mM ABTS.
BHAL pH optimum
To determine the optimal experimental setup for BHAL activity measurements, the best pH had to be determined. Using Britton-Robinson buffer pHs between pH 4 and pH 9 had been adjusted. 308 ng BHAL per well had been tested under these pH conditions using 7 mM ABTS. The CuCl2 incubated and therefor activated BHAL showed a high activity at pH 4 and pH 5, where most of ABTS was oxidized (compared to Fig. 8 and 9). The calculated specific enzyme activity of BHAL showed high activity at both mentioned pHs (Fig. 10). While BHAL had an activity of ~8 U mg-1 at pH 4 and pH 5, the enzyme activity decreased at higher pHs. At a pH of 6 only 1/3 of enzyme activity could be detected compared to the activity at pH 4 and pH 5. While still active at pH 7, the BHAL is not as suitable as thought for an application at a waste water treatment plant because of its high activity in acidic environments.
BHAL activity at different temperatures
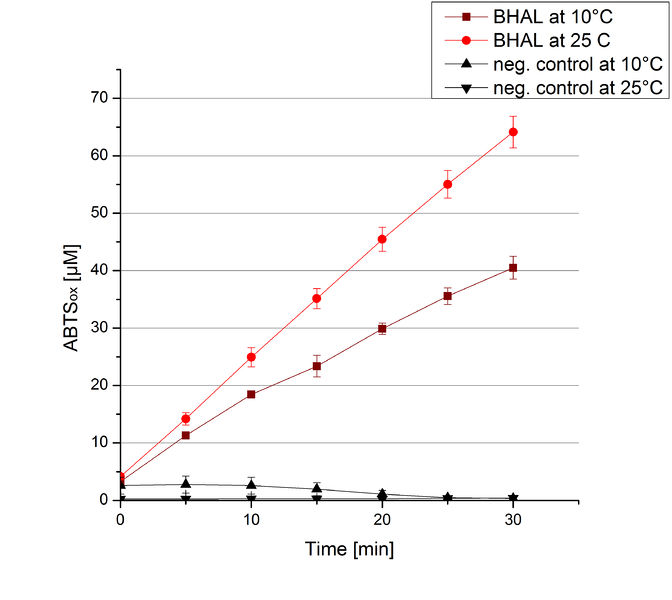
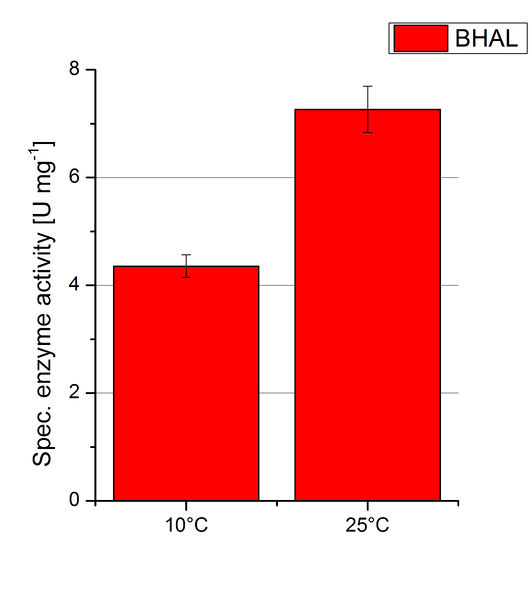
To investigate the activity of BHAL at temperatures that will apply at a waste water treatment plant throughout the year, activity tests were performed at 10 °C and 25 °C as described above. The measurements were conducted for 30 minutes. The obtained results revealed a lower activity of BHAL at 10 °C in comparison to 25 °C (see Fig. 11). The obtained results were used to calculate the specific enzyme activity which was at 4.2 and 7.2 U mg-1, respectively (see Figure 12). The negative control without BHAL but 0.4 mM CuCl2 at 10 °C and 25 °C showed a negligible oxidation of ABTS. The activity of BHAL was increased to about 60 % at 10 °C but nevertheless the observed activity at both conditions was great news for the possible application in waste water treatment plants.
Substrate Analysis
The measurements were made to test if the produced laccases were able to degrade different hormones. Therefore the produced laccases were inserted in the same concentrations (3 µg mL-1) to the different measurement approaches. To work with the correct pH value (which were measured by the Team Activity Test) Britton Robinson buffer at pH 5 was used for all measurements. The initial substrate concentration was 5 µg mL-1. The results of the reactions without ABTS are shown in Figure 2. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are indicated. The X-axis displays the different tested laccases. The degradation was measured at t0 and after five hours of incubation at 30 °C. The negative control was the substrate in Britton Robinson buffer and showed no degradation of the substrates. The bought laccase TVEL0 which is used as positive control is able to degrade 94.7 % estradiol and 92.7 % ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 35.9 % of used estradiol after five hours. ECOL was able to degrade 16.8 % estradiol. BHAL degraded 30.2 % estradiol. The best results were determined with TTHL (laccase from Thermus thermophilus). Here the percentage of degradation amounted 55.4 %.
The results of the reactions of the laccases with addition of ABTS are shown in Figure 3. The experimental set ups were the same as the reaction approach without ABTS described above. The X-axis displays the different tested laccases. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are shown. The degradation was measured at t0 and after five hours of incubation at 20 °C. The negative control showed no degradation of estradiol. 6.8 % of ethinyl estradiol was decayed. The positive control TVEL0 is able to degrade 100 % estradiol and ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 46.9 % of used estradiol after ten minutes incubation. ECOL was able to degrade 6.7 % estradiol. BHAL degraded 46.9 % estradiol. With TTHL (laccase from Thermus thermophilus)a degradation 29.5 % were determined.
Immobilization
Figure 20 shows the percentage of laccases bound after incubation with CPC-beads, relative to the original concentration. The concentration of laccases in the supernatant after incubation was measured using Roti®-Nanoquant. The results showed that only 21% of BHAL laccases was still present in the supernatant. This illustrates that BHAL was successfully immobilized on the CPC-beads.
Figure 22 shows the illustration of ABTS oxidation by BHAL with time compared to the negative control. The increase in ABTS oxidized proves laccase activity even if a direct comparison with the original and not immobilized laccase solution was not possible due to the very low concentration of purified BHAL.