Difference between revisions of "Part:BBa K771206"
ChobitParrot (Talk | contribs) |
Zhiantinglan (Talk | contribs) (→Violacein synthetic pathway) |
||
| Line 13: | Line 13: | ||
The violacein biosynthetic pathway includes 5 key enzymes which work in conjunction. VioA, a flavoenzyme and VioB, a heme protein, work together to oxidize and dimerize tryptophen into IPA imine dimer. Then vioE induces a indole rearrangement,producing prodeoxyviolacein acid, also known as PVA. | The violacein biosynthetic pathway includes 5 key enzymes which work in conjunction. VioA, a flavoenzyme and VioB, a heme protein, work together to oxidize and dimerize tryptophen into IPA imine dimer. Then vioE induces a indole rearrangement,producing prodeoxyviolacein acid, also known as PVA. | ||
| − | [[Image: | + | [[Image:FigI-SJTU violacein-pathway.png|400px|center|The violacein biosynthetic pathway]] |
VioA, VioB and VioE catalyze a sequential reaction to produce PVA. There exists one intrinsic E.coli enzyme that aids in an additional side reaction, further modifying PVA into a green pigment called deoxychromoviridans. | VioA, VioB and VioE catalyze a sequential reaction to produce PVA. There exists one intrinsic E.coli enzyme that aids in an additional side reaction, further modifying PVA into a green pigment called deoxychromoviridans. | ||
Latest revision as of 11:27, 26 October 2012
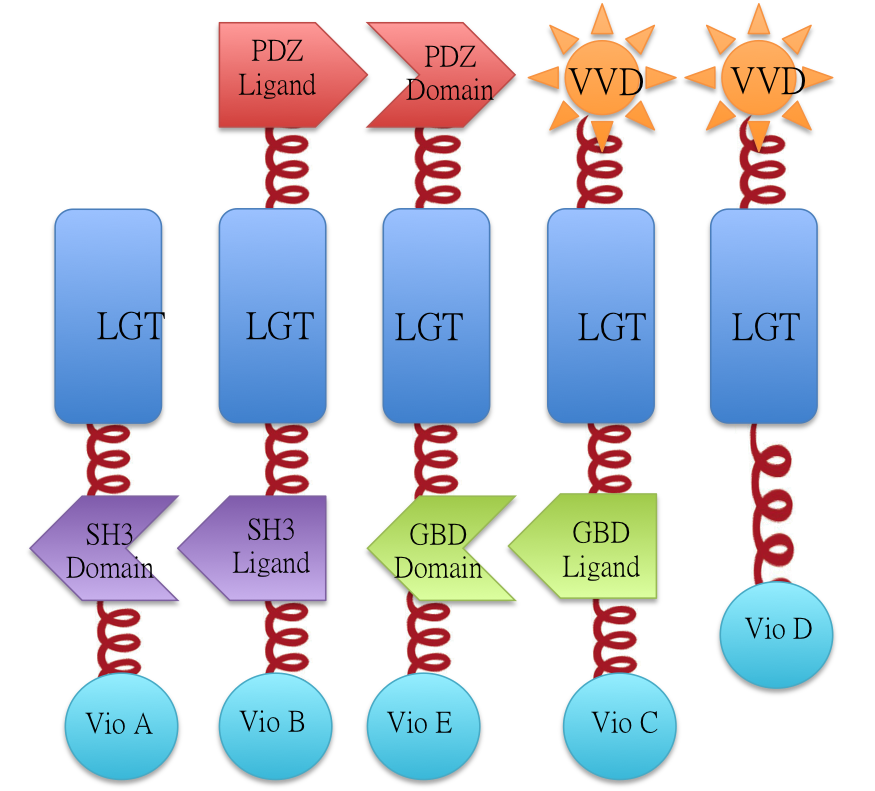
Vio D with Membrane Anchor 6
This part is composed with two parts, the Membrane Anchor 6 and Violacein synthetic pathway enzyme vioD. The enzyme can be aggregated with other membrane anchors, composing the Membrane Magic system. For more information, please visit [http://2012.igem.org/Team:SJTU-BioX-Shanghai 2012 SJTU-BioX-Shanghai wiki]
Background
Violacein is a violet pigment produced by several bacteria through pathway of 5 related genes, vioA, vioB, vioC, vioD and vioE. It was initially discovered in Chromobacterium violaceum. This metabolite of tryptophan has such special applications as antibacterial, anti-trypanocidal, anti-ulcerogenic, and anticancer drugs. There are three branches in this pathway which could verify the efficiency of the Membrane Rudder.
Violacein synthetic pathway
The violacein biosynthetic pathway includes 5 key enzymes which work in conjunction. VioA, a flavoenzyme and VioB, a heme protein, work together to oxidize and dimerize tryptophen into IPA imine dimer. Then vioE induces a indole rearrangement,producing prodeoxyviolacein acid, also known as PVA.
VioA, VioB and VioE catalyze a sequential reaction to produce PVA. There exists one intrinsic E.coli enzyme that aids in an additional side reaction, further modifying PVA into a green pigment called deoxychromoviridans.
The last two enzymes, VioC and VioD are flavin-dependent oxygenases. VioC alone transforms PVA into a purple pigment called deoxyviolacein, while Vio C could also act cooperatively with VioD. VioD hydroxylates 5-position indole ring, then the other 2-position indole ring is processed by VioC to create the oxindole, and in this way violacein is produced.
Design of Experiment
Switch of reaction direction
We constructed our device as demonstrated in Fig.2. In Violacein Membrane Rudder System, VioA, VioB, VioE and VioC constitutively aggregate. When blue light is present, VioD with Membrane Anchor would aggregate with complex of VioA,B,C and E, making violacein the dominnant final product. But if you turn the light off, VioD with Membrane Anchor would dissociate with VioA,B,C and E. Thus the biosynthetic pathway for deoxyviolacein is switched on.
For VioB and VioE only function normally in dimereic state, free VioB and free VioE were coexpressed with membrane assembly carrying VioA,B,C,D and E to ensure the normal function of the whole system.
To characterize the practical effect of Violacein Membrane Rudder System, we coexpressed all proteins in Fig.2 with free VioB and VioE. Bacteria in experimental group were induced at a L-Arabinose concentration of 0.1% under blue light while control group were induced in the dark with other conditions all the same.
Suppression of side-product
According to the mechanism behind our design of membrane rudder, a considerable reduction of deoxychromoviridans production is expected. Under light signal, VioD and VioC get close to other Vio enzymes. The assembly of sequential enzymes helps substrate flow to downstream enzymes, making violacein more preferably synthesized . The overall amount of available tryptophan in a bacterium is fixed, so preference to the production of violacein would inevitably cut the supply to the other branches of reaction.
To verify our assumption, free VioABCDE genes are also transformed into E.coli as control group.
Results and Discussion
Overview
By attaching the core enzymes to the VIVID, we managed to control the flow of branched chain reaction, such as violacein biosynthetic pathway. Results indicate that the extracts of bacterial cultures induced by light contained almost no deoxyviolacein while it was produced in the samples restrained from light. Moreover, a significant decrease of side products such as deoxychomoviridans was detected when membrane complex system was present.
Switch Analysis
The switch of the reaction can be realized by the extracellular signals. Through the light induction, the reaction producing deoxyviolacein by VioC alone is inhibited due to the lack of interaction with PVA. On the other hand, as long as light is restrained from the bacteria, however, the above reaction is initiated leading to the production of deoxyviolacein.The HPLC results show that the peak of deoxyviolacein appears at about 9 minutes after the injection. We find out that under the induction of light, production of deoxyviolacein was inhibited (almost 0%) while most products were violacein. Without the light induction, however, the pathway leading to the deoxyviolacein was initiated and thus deoxyviolacein was produced(about 8%). Such results confirmed our membrane rudder system and indicated that by controlling the extracellular signal, such as light, we are able to manipulate the branched chain reactions thus producing the target products we desire.
Suppression of side reaction
<br\>
Membrane complex system would help to reduce the amount of side-products to some extent. Under the case that the enzymes involved in the side reaction are situated in the cytoplasm, they are less competent than the core enzymes attached on the membrane to mediate the subsequent reactions due to the spatial obstacle.
From the HPLC results, we detected a distinct decrease of side-products deoxychomoviridans. Judging from the peak area, we find out that the amount of deoxychromoviridans from rbs-vio control group is nearly eight times that from light-induced group. Such experiment results coincided with our anticipation and demonstrated that the membrane complex system would enhance the usage of substrates and thus reduce the amount of intermediate products and other subsequent side-products.
<br\><br\><br\><br\><br\>
Violacein Protocols
Induction and Extraction
- Incubate the monoclonal from medium in a shaker at 37°C overnight
- Add 0.1% Arabinose, 1mM Ferrous ammonium sulfate and 40 μM to start induction for another 6 hours
- Centrifuge for 1 min at 10000rpm, remove the LB Medium
- Add 800 μL 10%SDS and break cells using ultrasound
- Add 800 μL ethyl acetate to extract violacein/deoxyviolacein
- Use rotovap to concentrate violacein and resuspend with methanol
HPLC
- Column: C18, 15cm
- Mobile phase: 75% methanol, 25% ddH2O
- Injection volume: 20 μL
- Speed: 0.5 mL/min
- Detection: 230 nm
HPLC Data
Related Parts
VioA with Membrane Anchor 2 (BBa_K771201)
VioB with Membrane Anchor 3 (BBa_K771202)
VioE with Membrane Anchor 4 (BBa_K771203)
VioC with Membrane Anchor 5 (BBa_K771204)
VIoC with Membrane Anchor 5 (BBa_K771205)
Viod with Membrane Anchor 6 (BBa_K771206)
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 1465
Illegal XbaI site found at 1477 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 1465
- 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 1465
- 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 1465
Illegal XbaI site found at 1477 - 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 1465
Illegal XbaI site found at 1477
Illegal AgeI site found at 494
Illegal AgeI site found at 1339 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 960
Illegal SapI.rc site found at 2458
Illegal SapI.rc site found at 2533