Difference between revisions of "Part:BBa J23104:Experience"
(→Characterization experiment by qrtPCR on BBa_J23100, BBa_J23104, BBa_J23105, BBa_J23106, BBa_J23109, BBa_J23112, BBa_J23113, BBa_J23114 by iGEM Team Göttingen (by C. Krüger and J. Kampf)) |
|||
| Line 33: | Line 33: | ||
=====Description===== | =====Description===== | ||
| − | We used quantitative real-time PCR as a powerful tool for | + | We used quantitative real-time PCR as a powerful tool for quantification of gene expression. We used this method to examine the expression rate of the ''Tar'' receptor gene under control of promoters from the [https://parts.igem.org/Promoters/Catalog/Anderson Anderson family] of the parts registry. The BioBricks (K777001-K777008) we used for this experiment can be found [https://parts.igem.org/Part:BBa_K777001 here]. |
| − | The reported activities of these promoters are given as the relative fluorescence of these plasmids in strain TG1 [https://parts.igem.org/Promoters/Catalog/Anderson]. Promoter constructs were cloned into the vector pSB1C3 and expressed in <i>E.coli</i> BL21DE3 grown in LB-media (lysogeny broth). The measurements were performed for each construct and reference as a triplet. Additionally, we included H<sub>2</sub>O as negative control to predict possible contamination. For the evaluation of our results, | + | The reported activities of these promoters are given as the relative fluorescence of these plasmids in strain TG1 [https://parts.igem.org/Promoters/Catalog/Anderson]. Promoter constructs were cloned into the vector pSB1C3 and expressed in <i>E.coli</i> BL21DE3 grown in LB-media (lysogeny broth). The measurements were performed for each construct and reference as a triplet. Additionally, we included H<sub>2</sub>O as negative control to predict possible contamination. For the evaluation of our results, the 2<sup>–ΔΔCT</sup> (Livak) method was applied. We used the weakest promoter with the lowest expression rate as calibrator for the calculations and as reference the housekeeping gene ''rrsD'' of <i>E.coli</i>. |
| − | You can find detailed information of the qrtPCR approach [http://2012.igem.org/Team:Goettingen/Project/Methods#-.3E_Experimental_design here].<br> | + | You can find detailed information of the qrtPCR approach [http://2012.igem.org/Team:Goettingen/Project/Methods#-.3E_Experimental_design here].<br><br> |
Revision as of 21:12, 26 September 2012
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_J23104
User Reviews
UNIQ134f8810b1bdf1d7-partinfo-00000000-QINU UNIQ134f8810b1bdf1d7-partinfo-00000001-QINU
|
•••••
for iGEM-Team Goettingen 2012 |
Characterization experiment by qrtPCR on BBa_J23100, BBa_J23104, BBa_J23105, BBa_J23106, BBa_J23109, BBa_J23112, BBa_J23113, BBa_J23114 by iGEM Team Göttingen (by C. Krüger and J. Kampf)DescriptionWe used quantitative real-time PCR as a powerful tool for quantification of gene expression. We used this method to examine the expression rate of the Tar receptor gene under control of promoters from the Anderson family of the parts registry. The BioBricks (K777001-K777008) we used for this experiment can be found here. The reported activities of these promoters are given as the relative fluorescence of these plasmids in strain TG1 [1]. Promoter constructs were cloned into the vector pSB1C3 and expressed in E.coli BL21DE3 grown in LB-media (lysogeny broth). The measurements were performed for each construct and reference as a triplet. Additionally, we included H2O as negative control to predict possible contamination. For the evaluation of our results, the 2–ΔΔCT (Livak) method was applied. We used the weakest promoter with the lowest expression rate as calibrator for the calculations and as reference the housekeeping gene rrsD of E.coli.
You can find detailed information of the qrtPCR approach [http://2012.igem.org/Team:Goettingen/Project/Methods#-.3E_Experimental_design here].
Results & Discussion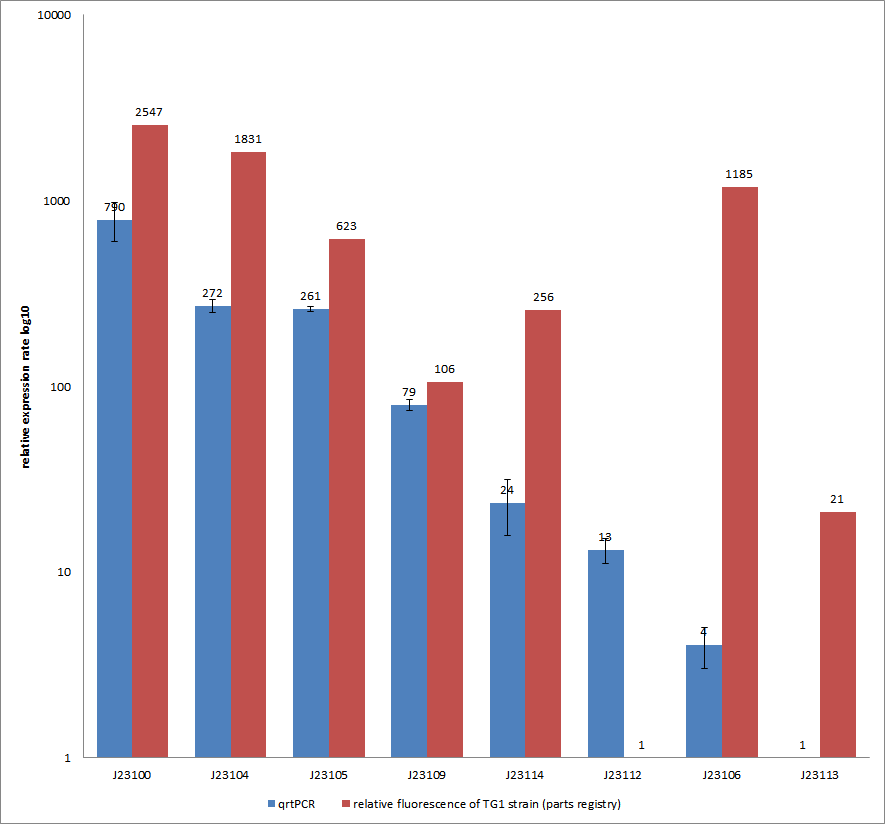 Comparison of relative expression rates of constitutive promoters by qrtPCR and relative fluorescence (see parts registry,Anderson family). The blue bar indicates the measured expression rates for our constructs (J23100, J23104, J23105, J23106, J23109, J23112, J23113, J23114) and the red ones those for the literature values represented in the “parts registry”. The measurements are illustrated in a logarithmic application. The standard variation was calculated for our measured values (black error bar). As mentioned before, both datasets were collected by methods which produce data at different points after the gene expression. Quantitative real time PCR measures the amount of expressed mRNA while relative fluorescence measurements quantify on protein level. In perspective of stability and half-life periods of mRNA and proteins or due to protein modification, it is comprehensible to obtain varying data-sets and expression rates. Another problem that occurred during our quantitative real-time measurements was the deviation in some of biological replicates. This problem was also observed in another group’s experiments ([http://www.jbioleng.org/content/3/1/4 Kelly et al., 2009]). They mentioned variations across experimental conditions in the absolute activity of the BioBricks. To reduce variation in promoter activity, they measured the activity of promoters relative to BBa_J23101. Furthermore, the iGEM team of Groningen which participated in 2009 also measured the relative fluorescence of TG1 strain with the promoters J23100, J23109 and J23106 via Relative Promoter Units (RPUs). Their values indicated the comparable tendency to our documented values |
