Difference between revisions of "BBa K731700 and BBa K731710 measurements"
(→PROTOCOL DEVELOPMENT:) |
|||
| Line 52: | Line 52: | ||
'''Emission and Excitation parameter choise:''' | '''Emission and Excitation parameter choise:''' | ||
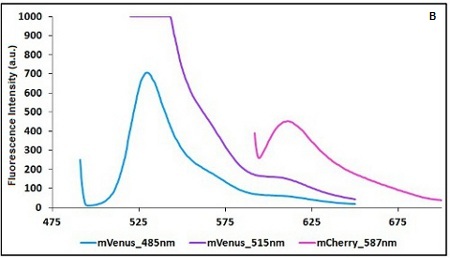
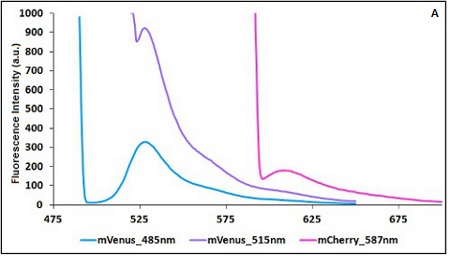
| − | The emission and the excitation parameter must | + | The emission and the excitation parameter must be aimed at achieving the maximum distance between the two point at which you take emission measurements and the more comparability in terms of fluorescence intensity accordingly to the limits imposed by the two fluorescent proteins in use. |
[[Image:Wls_K731710_520V.jpg]] [[Image:Wls_K731700_570V.jpg]] | [[Image:Wls_K731710_520V.jpg]] [[Image:Wls_K731700_570V.jpg]] | ||
Revision as of 09:31, 22 September 2012
Hello world
In this page we are really proud to introduce you the protocol we (Giacomo and Anna) developed for the characterization transcriptional terminators effects on gene expression. (Tested on BBa_J64997)
The develop of this protocolcost us not only sleepless night but also sunny and beautiful weekend of trekking so take a hot cup of tea and read it all.
IN VIVO ANALYSIS
|
BEFORE STARTING:
NOTE1:Antibiotic are at concentration of 0.1mg/ml ampicillin and 0.035mg/ml cloranphenicol NOTE2:Four glycerol stocks for both control and sample will give you a good level of significance
|
PROTOCOL DEVELOPMENT:
Emission and Excitation parameter choise:
The emission and the excitation parameter must be aimed at achieving the maximum distance between the two point at which you take emission measurements and the more comparability in terms of fluorescence intensity accordingly to the limits imposed by the two fluorescent proteins in use.