Difference between revisions of "Part:BBa K525123"
(→Identification and localisation) |
(→Identification and localisation) |
||
| Line 34: | Line 34: | ||
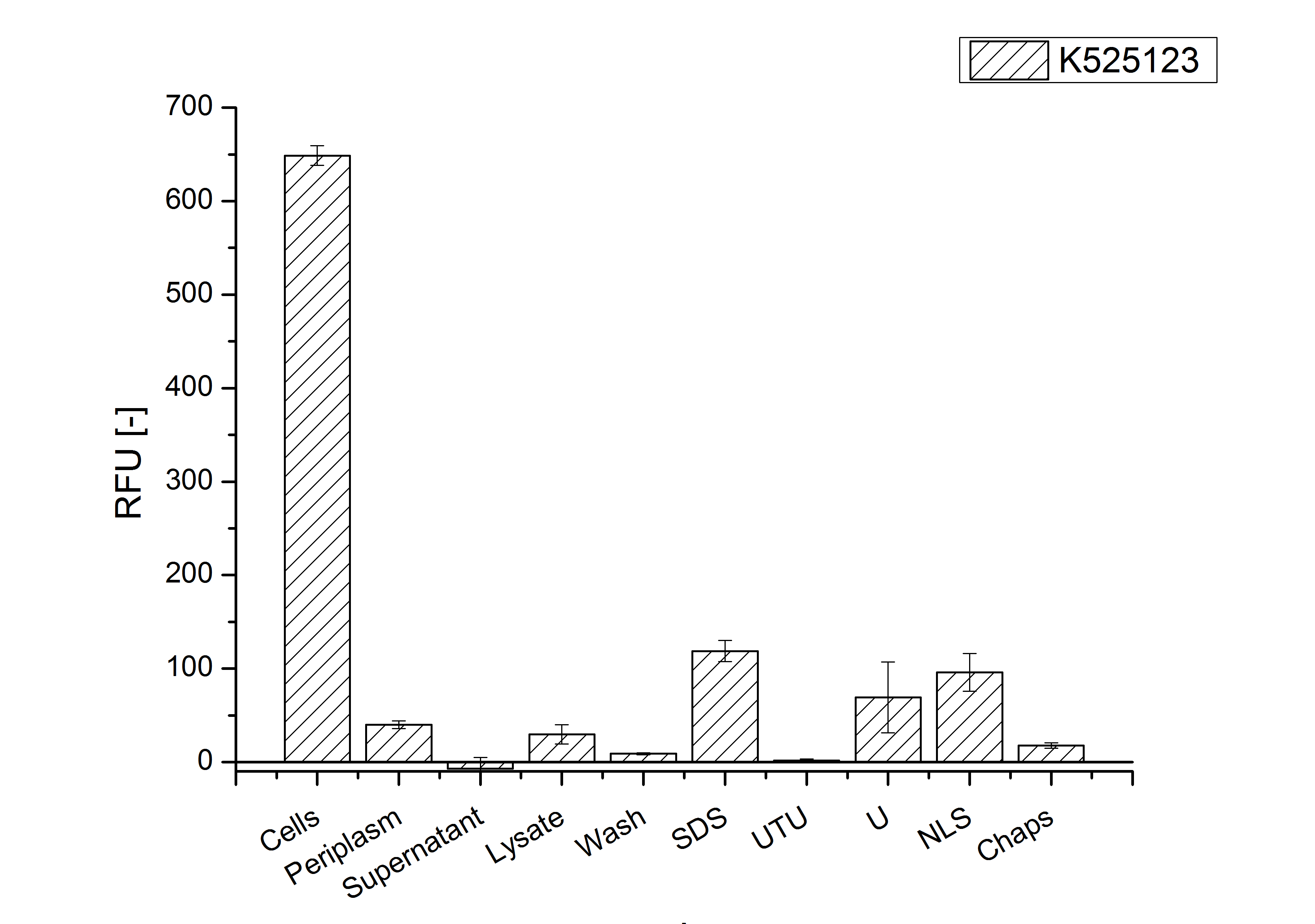
An other important fact is, that there is actually mRFP fluorescence measurable in such high concentrated detergent solutions. The S-layer seems to stabilize the biologically active conformation of mRFP. The MALDI-TOF analysis of the relevant size range in the polyacrylamid gel approved the existance of the intact fusion protein in all detergent fractions (fig x). | An other important fact is, that there is actually mRFP fluorescence measurable in such high concentrated detergent solutions. The S-layer seems to stabilize the biologically active conformation of mRFP. The MALDI-TOF analysis of the relevant size range in the polyacrylamid gel approved the existance of the intact fusion protein in all detergent fractions (fig x). | ||
| − | [[Image:Bielefeld 2011 BF3 Purification.png|700px|thumb|center| '''Figure 3: Fluorescence pro OD<sub>600</sub> progression of the CspB/mRFP [https://parts.igem.org/Part:BBa_E1010 (BBa_E1010)] fusion protein initiating with the cultivation fractions up to the detergent fractions of the seperate denaturations. Cultivations were carried out in autoinduction medium at 37 ˚C. The cells were mechanically disrupted and the resulting biomass was wahed with ddH<sub>2</sub>O and resuspendet in the respective detergent. The used detergent acronyms stand for: SDS = 10 % | + | [[Image:Bielefeld 2011 BF3 Purification.png|700px|thumb|center| '''Figure 3: Fluorescence pro OD<sub>600</sub> progression of the CspB/mRFP [https://parts.igem.org/Part:BBa_E1010 (BBa_E1010)] fusion protein initiating with the cultivation fractions up to the detergent fractions of the seperate denaturations. Cultivations were carried out in autoinduction medium at 37 ˚C. The cells were mechanically disrupted and the resulting biomass was wahed with ddH<sub>2</sub>O and resuspendet in the respective detergent. The used detergent acronyms stand for: SDS = 10% sodium dodecyl sulfate; UTU = 7 M urea and 3 M thiourea; U = 10 M urea; NLS = 10% n-lauroyl sarcosine; 2% CHAPS = 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate.''']] |
<!-- --> | <!-- --> | ||
Revision as of 19:33, 21 September 2011
S-layer cspB from Corynebacterium glutamicum with lipid anchor and PT7 and RBS
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1334
Illegal XhoI site found at 161
Illegal XhoI site found at 779 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1313
Illegal SapI site found at 560
Illegal SapI site found at 772
Illegal SapI site found at 1320
Expression in E. coli
For characterization the CspB gen was fused with a monomeric RFP (BBa_E1010) using Gibson assembly.
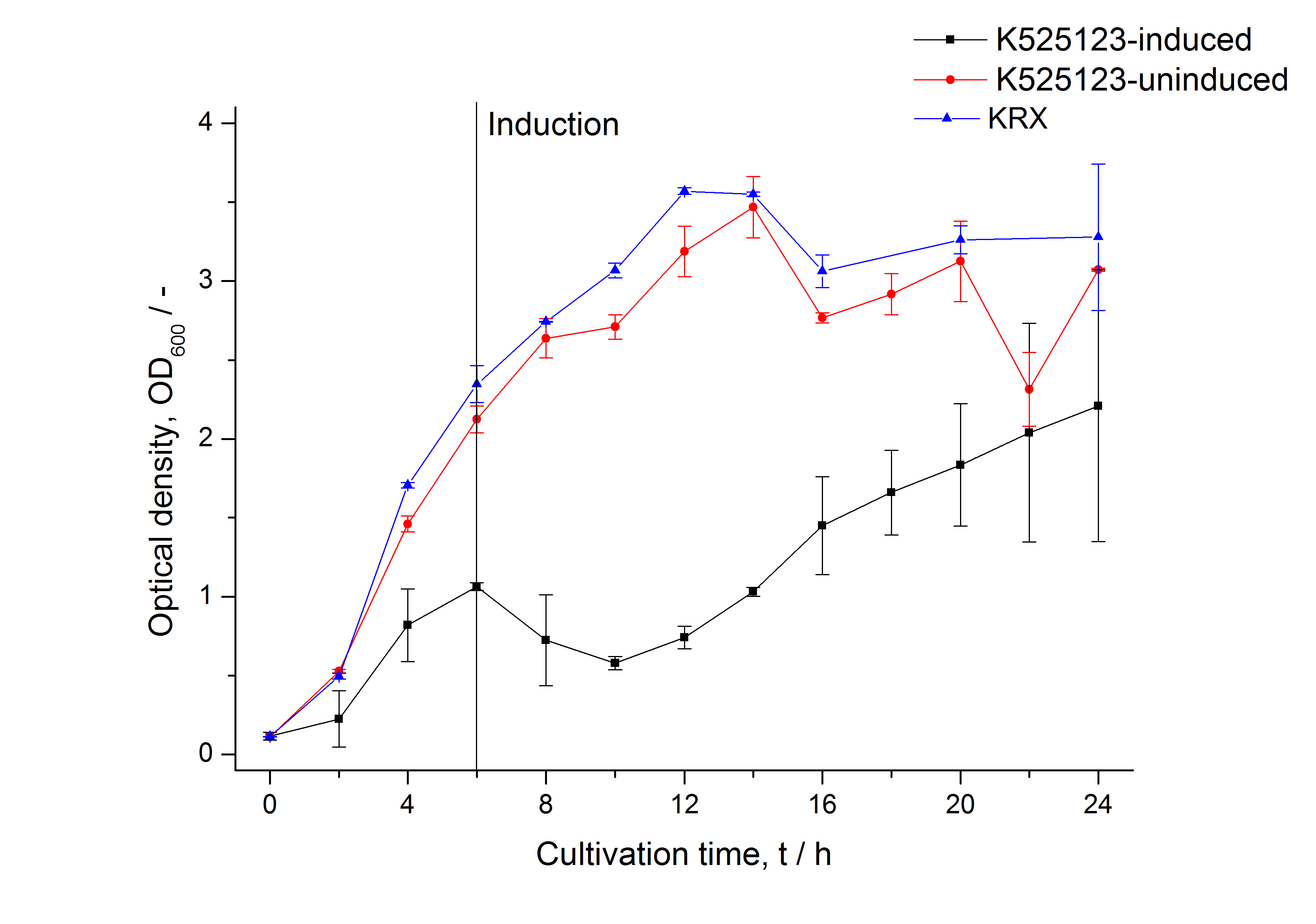
The CspB|mRFP fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0,1 % L-rhamnose using the autinduction protocol from promega.
Identification and localisation
After a cultivation time of 18 h the CspB|mRFP fusion protein has to be localized in E. coli KRX. Therefor a part of the produced biomass was mechanically disrupted and the resulting lysate was wahed with ddH2O. From the other part the periplasm was detached by using a osmotic shock. The existance of fluorescene in the periplasma fraction, showed in fig. 3, indicates that Brevibacterium flavum TAT-signal sequence is at least in part functional in E. coli KRX.
The S-layer fusion protein could not be found in the polyacrylamide gel after a SDS-PAGE of the lysate. This indicated that the fusion protein intigrates into the cell membrane with its lipid anchor. For testing this assumption the washed lysate was treted with ionic, nonionic and zwitterionic detergents to release the CspB|mRFP out of the membranes.
The existance of flourescence in the detergent fractions and the proportionally low fluorescence in the wash fraction confirm the hypothesis an insertion into the cell membrane (fig. 3). An insertion of these S-layer proteins might stabilize the membrane structure and increase the stability of cells against mechanical and chemical treatment. A stabilization of E. coli expressing S-lyer proteins was discribed by Lederer et al., (2010).
An other important fact is, that there is actually mRFP fluorescence measurable in such high concentrated detergent solutions. The S-layer seems to stabilize the biologically active conformation of mRFP. The MALDI-TOF analysis of the relevant size range in the polyacrylamid gel approved the existance of the intact fusion protein in all detergent fractions (fig x).