Difference between revisions of "Part:BBa K404122"
| (15 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K404122 short</partinfo> | <partinfo>BBa_K404122 short</partinfo> | ||
| + | {| style="color:black; margin: 0px 0px 5px 20px;" cellpadding="6" cellspacing="1" border="2" align="center" | ||
| + | ! colspan="2" style="background:#66bbff;"|[https://parts.igem.org/Part:BBa_K404122 AAV2-left-ITR_pCMV_betaglobin_mGMK_TK30_hGH_AAV2-right-ITR] | ||
| + | |- | ||
| + | |'''BioBrick Nr.''' | ||
| + | |[https://parts.igem.org/Part:BBa_K404122 BBa_K404122] | ||
| + | |- | ||
| + | |'''RFC standard''' | ||
| + | |[https://parts.igem.org/Help:Assembly_standard_10 RFC 10] | ||
| + | |- | ||
| + | |'''Requirement''' | ||
| + | |pSB1C3<br> | ||
| + | |- | ||
| + | |'''Source''' | ||
| + | | | ||
| + | |- | ||
| + | |'''Submitted by''' | ||
| + | |[http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] | ||
| + | |} | ||
| − | |||
| − | |||
| − | |||
<html> | <html> | ||
| − | < | + | <h2>Contents</h2> |
| − | <p class="MsoToc2"><a href="#_Toc275981841"><span lang="EN-US"> | + | <p class="MsoToc2"><a href="#_Toc275981841"><span lang="EN-US">Functional Composite Part for Successful Tumor Cell Ablation</span><span style="color: windowtext; display: none; text-decoration: none;">. </span><span style="color: windowtext; display: none; text-decoration: none;">1</span></a></p> |
| − | + | ||
<p class="MsoToc3"><a href="#_Toc275981842"><span lang="EN-US">Introduction</span><span style="color: windowtext; display: none; text-decoration: none;">. </span><span style="color: windowtext; display: none; text-decoration: none;">1</span></a></p> | <p class="MsoToc3"><a href="#_Toc275981842"><span lang="EN-US">Introduction</span><span style="color: windowtext; display: none; text-decoration: none;">. </span><span style="color: windowtext; display: none; text-decoration: none;">1</span></a></p> | ||
| Line 26: | Line 40: | ||
Ganciclovir Concentrations for Efficient Cell Killing by Cytotoxicity Assays</span><span style="color: windowtext; display: none; text-decoration: none;">. </span><span style="color: windowtext; display: none; text-decoration: none;">8</span></a></p> | Ganciclovir Concentrations for Efficient Cell Killing by Cytotoxicity Assays</span><span style="color: windowtext; display: none; text-decoration: none;">. </span><span style="color: windowtext; display: none; text-decoration: none;">8</span></a></p> | ||
| − | |||
| − | |||
<p class="MsoToc3"><a href="#_Toc275981848"><span lang="EN-US">Conclusions</span><span style="color: windowtext; display: none; text-decoration: none;">. </span><span style="color: windowtext; display: none; text-decoration: none;">16</span></a></p> | <p class="MsoToc3"><a href="#_Toc275981848"><span lang="EN-US">Conclusions</span><span style="color: windowtext; display: none; text-decoration: none;">. </span><span style="color: windowtext; display: none; text-decoration: none;">16</span></a></p> | ||
| Line 35: | Line 47: | ||
<p class="MsoNormal"> </p> | <p class="MsoNormal"> </p> | ||
| − | <h2><a name="_Toc275981841"><span lang="EN-US"> | + | <h2><a name="_Toc275981841"><span lang="EN-US">Functional Composite Part for Successful Tumor Cell Ablation</span></a></h2> |
<h3><a name="_Toc275981842"><span lang="EN-US">Introduction</span></a></h3> | <h3><a name="_Toc275981842"><span lang="EN-US">Introduction</span></a></h3> | ||
| Line 53: | Line 65: | ||
<p class="MsoNormal"><span lang="EN-US">The iGEM team Freiburg_Bioware 2010 provides | <p class="MsoNormal"><span lang="EN-US">The iGEM team Freiburg_Bioware 2010 provides | ||
both the cytosine deaminase (CD, BBa_K404112) and an improved guanylate kinase - | both the cytosine deaminase (CD, BBa_K404112) and an improved guanylate kinase - | ||
| − | thymidine kinase fusion gene (mGMK_TK, BBa_K404113) within the Virus | + | thymidine kinase fusion gene (mGMK_TK, <a href = "https://parts.igem.org/Part:BBa_K404113" target="blank" > BBa_K404113</a>) within the Virus |
Construction Kit as effective suicide genes. We demonstrate efficient and | Construction Kit as effective suicide genes. We demonstrate efficient and | ||
specific killing of tumor cells by enzymatic cytotoxicity assays, flow | specific killing of tumor cells by enzymatic cytotoxicity assays, flow | ||
| Line 66: | Line 78: | ||
of the constructs carrying the suicide genes was performed following the | of the constructs carrying the suicide genes was performed following the | ||
BioBrick Standard Assembly. All plasmids contain the enhancer-element human <i>beta-globin</i> | BioBrick Standard Assembly. All plasmids contain the enhancer-element human <i>beta-globin</i> | ||
| − | intron (BBa_K404107) and the <i>human growth hormone</i> terminator signal (hGH, | + | intron (<a href = "https://parts.igem.org/Part:BBa_K404107" target="blank" > BBa_K404107</a>) and the <i>human growth hormone</i> terminator signal (hGH, |
| − | BBa_K404108) flanked by the inverted terminal repeats (ITRs, BBa_K404100 and | + | <a href = "https://parts.igem.org/Part:BBa_K404108" target="blank" > BBa_K404108</a>) flanked by the inverted terminal repeats (ITRs, <a href = "https://parts.igem.org/Part:BBa_K404100" target="blank" > BBa_K404100</a> and |
| − | BBa_K404101). Assembled suicide genes are either under the control of the CMV | + | <a href = "https://parts.igem.org/Part:BBa_K404101" target="blank" > BBa_K404101</a>). Assembled suicide genes are either under the control of the CMV |
| − | promoter (BBa_J52034) or the tumor-specific telomerase promoter phTERT (BBa_K404106). | + | promoter (<a href = "https://parts.igem.org/Part:BBa_J52034" target="blank" > BBa_J52034</a>) or the tumor-specific telomerase promoter phTERT (<a href = "https://parts.igem.org/Part:BBa_K404106" target="blank" > BBa_K404106</a>). |
</span></p> | </span></p> | ||
| Line 85: | Line 97: | ||
</tr> | </tr> | ||
</tbody></table> | </tbody></table> | ||
| + | |||
| + | </html> | ||
| + | [[Image:Freiburg10_Vectorplasmid composite 4.png|thumb|center|480px]]<br> | ||
| + | <html> | ||
| + | |||
</div> | </div> | ||
| Line 91: | Line 108: | ||
<p class="MsoNormal"><span lang="EN-US">To enable modularization of the thymidine | <p class="MsoNormal"><span lang="EN-US">To enable modularization of the thymidine | ||
| − | kinase mutants TK30 and SR39 (BBa_K404109 and BBa_K404110) according to the | + | kinase mutants TK30 and SR39 (<a href = "https://parts.igem.org/Part:BBa_K404109" target="blank" > BBa_K404109</a> and <a href = "https://parts.igem.org/Part:BBa_K404110" target="blank" > BBa_K404110</a>) according to the |
| − | BioBrick standard, the fusion genes mGMK_TK30 and mGMK_SR39 (BBa_K404113 and </span><span style="line-height: 200%; color: black;" lang="EN-US">BBa_K404315</span><span lang="EN-US">) and CD (</span><span style="line-height: 200%; color: black;" lang="EN-US">BBa_K404112</span><span lang="EN-US">) were modified using the QuikChange | + | BioBrick standard, the fusion genes mGMK_TK30 and mGMK_SR39 (<a href = "https://parts.igem.org/Part:BBa_K404113" target="blank" > BBa_K404113</a> and </span><span style="line-height: 200%; color: black;" lang="EN-US"><a href = "https://parts.igem.org/Part:BBa_K404315" target="blank" > BBa_K404315</a></span><span lang="EN-US">) and CD (</span><span style="line-height: 200%; color: black;" lang="EN-US"><a href = "https://parts.igem.org/Part:BBa_K404112" target="blank" > BBa_K404112</a></span><span lang="EN-US">) were modified using the QuikChange |
Lightning Site-Directed Mutagenesis Kit (Stratagene) for deletion of iGEM RFC10 | Lightning Site-Directed Mutagenesis Kit (Stratagene) for deletion of iGEM RFC10 | ||
| − | pre- and suffix restriction sites. </span><span lang="EN-US">Figure | + | pre- and suffix restriction sites. </span><span lang="EN-US">Figure 2</span><span lang="EN-US"> demonstrates one example of |
successful deletion of a PstI restriction site located within the mGMK_TK30 | successful deletion of a PstI restriction site located within the mGMK_TK30 | ||
sequence at position 3109. A point mutation was introduced replacing the | sequence at position 3109. A point mutation was introduced replacing the | ||
| Line 122: | Line 139: | ||
vector plasmids was performed. An example for the last assembly step of | vector plasmids was performed. An example for the last assembly step of | ||
mGMK_TK30 and hGH_rITR is shown in </span><span lang="EN-US">Figure 3</span><span lang="EN-US">. The plasmids were digested with | mGMK_TK30 and hGH_rITR is shown in </span><span lang="EN-US">Figure 3</span><span lang="EN-US">. The plasmids were digested with | ||
| − | both XbaI and PstI (New England Biolabs, Insert: BBa_K404116: hGH_rITR) or SpeI | + | both XbaI and PstI (New England Biolabs, Insert: <a href = "https://parts.igem.org/Part:BBa_K404116" target="blank" > BBa_K404116</a>: hGH_rITR) or SpeI |
and PstI (Vector) and loaded on an agarose gel. As demonstrated in the preparative | and PstI (Vector) and loaded on an agarose gel. As demonstrated in the preparative | ||
gel in </span><span lang="EN-US">Figure 3</span><span lang="EN-US">, the expected | gel in </span><span lang="EN-US">Figure 3</span><span lang="EN-US">, the expected | ||
| Line 222: | Line 239: | ||
nascent DNA chain leading to replication termination and finally resulting in | nascent DNA chain leading to replication termination and finally resulting in | ||
death of dividing cells. </span></p> | death of dividing cells. </span></p> | ||
| − | + | <br /> | |
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
<h3><a name="_Toc275981845"><span lang="EN-US">Quantitative Analysis of Cell | <h3><a name="_Toc275981845"><span lang="EN-US">Quantitative Analysis of Cell | ||
Death by Flow Cytometry</span></a></h3> | Death by Flow Cytometry</span></a></h3> | ||
| Line 376: | Line 396: | ||
was plotted against increasing ganciclovir concentrations. </span></p> | was plotted against increasing ganciclovir concentrations. </span></p> | ||
| − | |||
| − | |||
| − | < | + | <h3><a name="_Toc275981848"><span lang="EN-US">Conclusions</span></a></h3> |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | <p class="MsoNormal"><span lang="EN-US"> | + | <p class="MsoNormal"><span lang="EN-US">Efficient and tissue-specific tumor killing |
| + | is one major challenge in cancer therapy </span><span lang="EN-US">(Black et al. 1996)</span><span lang="EN-US">. Gene-directed enzyme | ||
| + | prodrug therapy (GDEPT) is based on the conversion of non-toxic substances into | ||
| + | toxic drugs resulting in tumor cell death. The iGEM team Freiburg_Bioware 2010 | ||
| + | provides several functional suicide genes within the Virus Construction Kit, thus | ||
| + | offering a feasible and modular tool to the growing field of personalized | ||
| + | medicine and the iGEM community. We successfully demonstrated cancer cell death | ||
| + | caused by the introduction of modified fusion genes | ||
| + | consisting of guanylate and thymidine kinases.</span></p> | ||
| − | < | + | <p class="MsoNormal"><span lang="EN-US"> To prevent systemic toxic side effects of |
| + | conventional chemotherapy, the iGEM team Freiburg_Bioware 2010 took a leap and | ||
| + | efficiently retargeted the viral vector for directed suicide gene delivery | ||
| + | towards tumor cells. Capsid engineering was successfully demonstrated by the | ||
| + | iGEM team Freiburg_Bioware 2010. Further details can be found under <a href="http://2010.igem.org/Team:Freiburg_Bioware/Project/Results#targeting">iGEM wiki Freiburg_Bioware 2010 - Results - Targeting</a> | ||
| + | </span></p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | <p | + | <p><span lang="EN-US"> </span></p> |
| + | </html> | ||
| + | <h2>Individual components of the assembled vector plasmid </h2> | ||
| + | <h3>ITRs</h3><br> | ||
| + | <html> | ||
| + | <center> | ||
| + | <img src=https://static.igem.org/mediawiki/2010/8/88/Freiburg10_organization_ITRs.png width=400> | ||
| + | <p>Figure 10: ITR details </p> | ||
| + | </center> | ||
| + | </html><br> | ||
| + | The inverted terminal repeat structures can be subdivided into several palindromic motives: A and A’ form a stem loop which encases B and B’ as well as C and C’. Those motives form both arms of the T-shaped structure. The functional motives on the ITR are two regions that bind Rep 68/78, called Rep-binding elements (RBE on the stem and RBE’ on the B arm) and the terminal resolution site (trs) in which the rep proteins introduce single-stranded nicks. The 3’ OH end of the A motive acts as a primer for DNA replication (Im & Muzyczka, 1990) (Lusby, Fife, & Berns, 1980). | ||
| + | <h2>Beta-globin-intron</h2><br> | ||
| + | <html> | ||
| + | <center> | ||
| + | <img src=https://static.igem.org/mediawiki/2010/7/7c/Freiburg10_FACS_betaglobin.png width=400> | ||
| + | <p>Figure 11 A: FACS data </p> | ||
| + | </center> | ||
| + | </html><br> | ||
| + | <html> | ||
| + | <center> | ||
| + | <img src=https://static.igem.org/mediawiki/2010/2/26/Freiburg10_Diagram_betaglobin.png.png width=400> | ||
| + | <p>Figure 11 B: Calculated percentage of mVenus expressing cells</p> | ||
| + | </center> | ||
| + | </html><br> | ||
| − | + | The beta-globin intron BioBrick consists of a partial chimeric CMV promoter followed by the intron II of the beta-globin gene. The 3´end of the intron is fused to the first 25 bases of human beta globin gene exon 3. The beta globin intron BioBrick is assumed to enhance eukaryotic gene expression (Nott, Meislin, & Moore, 2003). As shown in Figure 11 A and B the vectorplasmid missing the beta-globin intron showed a negligible difference in mVenus expression compared to viral genomes containing the beta-globin intron. Considering these results and taking into account that a constant volume of viral particles has been used for transduction, the difference between the construct containing and lacking the beta-globin intron is minimal. Since packaging efficiency of the AAV-2 decreases with increasing sizes of the insert (Dong, Fan, & Frizzell, 1996), the iGEM team Freiburg_Bioware suggests using the beta-globin intron in dependence on the size of your transgene. | |
| − | AAV-2 | + | <h2>mGMK_TK30 fusion protein</h2><br> |
| − | The | + | The thymidine kinase mutant TK30 contains six modified amino acids (Black, Newcomb, Wilson, & Loeb, 1996) created in a first screening showing enhanced affinity for gancivlocir and acyclovir, but reduced specificity for its natural substrate thymidine.<br><br> |
| − | + | As efficient tumor killing and therefore ganciclovir activation is essential for successful tumor ablation, further improvements were conducted. Overexpression of transgenic thymidine kinase leads to accumulation of non-toxic intermediates, which cannot be phosphorylated sufficiently by endogenous guanylate kinase, the second enzyme in the salvage pathway of nucleotides. <br> | |
| + | Overcoming this bottleneck was accomplished by fusing the mouse guanylate kinase (mGMK) to the N-terminus of TK30 mutant creating a fusion protein (mGMK_TK30) with enhanced GCV/ACV sensitivity in vitro and in vivo (Ardiani, Sanchez-Bonilla, & Black, 2010) and improved bystander activity. The effect of non-transfected tumor cell killing upon transfer of toxic metabolites through gap junctional intercellular communication (GJIC) or immune-mediated tumor ablation is essential in suicide gene therapy (Pope, 1997). GCV-triphosphate is mainly transported through the central pore formed between connexin proteins from neighboring cells (Gentry, Im, Boucher, Ruch, & Shewach, 2005), but immune-induced bystander effect seems to be likely as well (Grignet-Debrus, Cool, Baudson, Velu, & Calberg-Bacq, 2000). <br> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | <html> | ||
| + | <h3> hGH termination signal</h3> | ||
| + | <p style="margin-right:100px" align="justify"> | ||
| + | Recombinant AAV genomes were engineered containing the inverted terminal repeats (ITRs), a strong eukaryotic promoter and mVenus as gene of interest with and without the hGH terminator signal. Transduction of HT1080 cells with viral particles containing the rAAV genomes and measuring mVenus expression 24-hours post infection by flow cytometry demonstrated that transgene expression of the constructs lacking the hGH termination signal is significantly reduced. The iGEM team Freiburg_Bioware 2010 therefore suggests using the provided hGH termination signal within the Virus Construction Kit for optimal gene expression. | ||
| + | </p> | ||
<p class="MsoNormal" style="text-indent: 0cm;"><span lang="EN-US"> </span></p> | <p class="MsoNormal" style="text-indent: 0cm;"><span lang="EN-US"> </span></p> | ||
| + | <p class="MsoNormal" style="text-align:justify;"><span lang="EN-US">Since cloning does not confirm | ||
| + | biological | ||
| + | activity, we analyzed the plasmids and their functional components, hGH | ||
| + | terminator and <i>beta-globin</i> intron, in cell culture. Assembled | ||
| + | plasmids | ||
| + | have been cotransfected, using AAV-293 cells, which provide the stable | ||
| + | integrated E1A and E1B genes, with helper plasmids required for capsid | ||
| + | assembly and genome encapsidation (pRC and pHelper) in a molar ratio of | ||
| + | 1:1:1 | ||
| + | (pGOI:pRC:pHelper). Virus particles containing the single stranded DNA | ||
| + | were | ||
| + | harvested 72-hours post transfection and HT1080 cells transduced with | ||
| + | constant | ||
| + | volumes of viral vectors. 48-hours post infection; transduced cells | ||
| + | expressing | ||
| + | the gene of interest were analyzed by flow cytometry.</span></p> | ||
| − | <p | + | </span><p style="text-align:justify;"> |
| − | the | + | Recombinant vectorplasmids |
| − | + | were engineered containing the inverted terminal repeats (ITRs), a | |
| − | + | strong | |
| + | eukaryotic promoter (CMV promoter: BBa_K404102) and mVenus as gene of | ||
| + | interest | ||
| + | with and without the hGH terminator signal. Transduction of HT1080 | ||
| + | cells with constant | ||
| + | volume of viral particles containing the vectorplasmids and measuring | ||
| + | mVenus | ||
| + | expression 24-hours post infection by flow cytometry demonstrated that | ||
| + | transgene expression of the constructs lacking the hGH termination | ||
| + | signal is | ||
| + | significantly reduced as shown in </span> | ||
| + | <span | ||
| + | lang="EN-US"> confirming the expected results | ||
| + | that hGH is essential for mRNA processing. The iGEM team | ||
| + | Freiburg_Bioware 2010 | ||
| + | therefore suggests using the provided hGH termination signal within the | ||
| + | Virus | ||
| + | Construction Kit for optimal gene expression.</p> | ||
| + | <table class="MsoTableGrid" | ||
| + | style="border: medium none ; border-collapse: collapse;" border="0" | ||
| + | cellpadding="0" cellspacing="0"> | ||
| + | </p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | < | + | <table> |
| + | <tr> | ||
| + | <td style="width: 400px; height: auto;"><img src="https://static.igem.org/mediawiki/parts/c/c7/Freiburg10_FACS_woHGH.png" style="width: 400px; height: auto;"/></td> | ||
| + | <td style="width: 400px;"><img src="https://static.igem.org/mediawiki/parts/7/70/Freiburg10_FACS_withHGH.png" style="width: 400px; height: auto;"/></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="width: 400px; vertical-align: top;">Figure 12: Flow cytometry analysis of vectorplasmid without (left) and with (right) hGH. For viral particle production, AAV-293 cells were transfected with the reassembled vector plasmid (BBa_K404119) or the reference plasmid, respectively.<br />A: Gating non transduced cells (control); subcellular debris and cellular aggreates can be distinguished from single cells by size, estimated forward scatter (FS Lin) and granularity, estimated side scatter (SS Lin) <br />B: Non-transduced cells plotted against cells expressing mVenus (Analytical gate was set such that 1% or fewer of negative control cells fell within the positive region (R5) <br />C: Gating transduced cells (R2 ≙ R14) (plasmids used for transfection: pGOI: pSB1C3_lITR_CMV_beta-globin_mVenus_hGH_rITR (pSB1C3_mVenus: BBa_K404119), pHelper, pRC. <br />D: Transduced cells plotted against cells expressing mVenus. R10 comprises transduced cells detected by mVenus fluorescence. <br />E: Overlay of non-transduced (red) and transduced (green).</td> | ||
| + | <td style="width: 400px; vertical-align: top;">F: Gating non-transduced cells (control). <br />G: Non-transduced cells plotted against cells expressing mVenus (R5). <br />H: Gating transduced cells (R14 ≙ R2) (plasmids used for transfection: pGOI: pAAV_mVenus, pHelper). <br />I: Transduced cells plotted against cells expressing mVenus. R10 comprises transduced cells detected by mVenus fluorescence. <br />J: Overlay of non-transduced (red) and transduced (green) cells plotted against mVenus expression. </td> | ||
| + | </tr> | ||
| + | </table> | ||
| − | < | + | <center> |
| − | + | <img src="https://static.igem.org/mediawiki/parts/9/94/Freiburg10_Diagram_hGH.png" style="width: 500px; height: auto; align: center; text-align: center;"/> <p>Figure 13: Calculated percentage of mVenus expressing cells </p> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | </ | + | <p style="align: center; text-align: center;">Flow cytometry analysis of vectorplasmids with and without hGH terminator. YFP expression of viral genomes was determined by flow cytomery after 24-hour post infection. Results demonstrate that mVenus expression of vectorplasmids lacking the hGH terminator is reduced significantly proving that the polyadenylation signal is essential for viral gene expression using recombinant viral vectors engineered by using components of the Virus Construction Kit.</p> |
| − | + | </center> | |
| − | < | + | </html> |
| − | + | <!-- Add more about the biology of this part here | |
| − | + | ===Usage and Biology=== | |
| − | + | ||
| − | < | + | <!-- --> |
| + | <span class='h3bb'>Sequence and Features</span> | ||
| + | <partinfo>BBa_K404122 SequenceAndFeatures</partinfo> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | < | + | <!-- Uncomment this to enable Functional Parameter display |
| − | + | ===Functional Parameters=== | |
| − | + | <partinfo>BBa_K404122 parameters</partinfo> | |
| − | + | <!-- --> | |
| − | + | ||
| − | + | ||
| + | <html> | ||
<h3><a name="_Toc275981849"><span lang="EN-US">References</span></a></h3> | <h3><a name="_Toc275981849"><span lang="EN-US">References</span></a></h3> | ||
| Line 546: | Line 571: | ||
http://www.ncbi.nlm.nih.gov/pubmed/8402637.</span></p> | http://www.ncbi.nlm.nih.gov/pubmed/8402637.</span></p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<p style="text-indent: 36pt;"><span style="font-size: 10pt; font-family: "Calibri","sans-serif";" lang="EN-US">Moolten, F.L., 1986. Tumor chemosensitivity | <p style="text-indent: 36pt;"><span style="font-size: 10pt; font-family: "Calibri","sans-serif";" lang="EN-US">Moolten, F.L., 1986. Tumor chemosensitivity | ||
| Line 566: | Line 584: | ||
prodrug-mediated cell killing. <i>Gene therapy</i>, 13(17), 1309-12. Available | prodrug-mediated cell killing. <i>Gene therapy</i>, 13(17), 1309-12. Available | ||
at: http://www.ncbi.nlm.nih.gov/pubmed/16810197.</span></p> | at: http://www.ncbi.nlm.nih.gov/pubmed/16810197.</span></p> | ||
| − | |||
| − | |||
</html> | </html> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 14:43, 30 October 2010
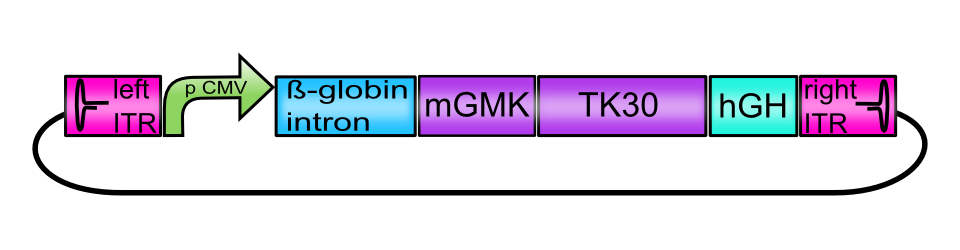
[AAV2]-left-ITR_pCMV_betaglobin_mGMK_TK30_hGH_[AAV2]-right-ITR
| AAV2-left-ITR_pCMV_betaglobin_mGMK_TK30_hGH_AAV2-right-ITR | |
|---|---|
| BioBrick Nr. | BBa_K404122 |
| RFC standard | RFC 10 |
| Requirement | pSB1C3 |
| Source | |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |
Contents
Functional Composite Part for Successful Tumor Cell Ablation
Successful Assembly of Vector Plasmids Carrying Suicide Genes via Cloning
Monitoring Efficient Tumor Killing by Phase-Contrast Microscopy
Quantitative Analysis of Cell Death by Flow Cytometry
Titrating Ganciclovir Concentrations for Efficient Cell Killing by Cytotoxicity Assays
Functional Composite Part for Successful Tumor Cell Ablation
Introduction
Gene delivery using viral vectors to specifically target tumor cells gained increasing attention in the last years being efficient in combination with suicide gene approaches (Willmon et al. 2006). Several prodrug/enzyme combinations have been reported. Two systems - ganciclovir (GCV)/herpes simplex virus thymidine kinase (HSV-TK) (Ardiani et al. 2010) and 5-fluorocytosine/cytosine deaminase (CD) (Fuchita et al. 2009a) – have been widely used and their therapeutic benefit was demonstrated in preclinical studies (Greco & Dachs 2001). Adeno-associated viruses (AAV) as delivery vectors are commonly used in suicide gene therapy. The suicide gene flanked by the inverted terminal repeats (ITRs) is encapsulated into the virus particles and delivered to the target cells where suicide gene expression is mediated by cellular proteins.
The iGEM team Freiburg_Bioware 2010 provides both the cytosine deaminase (CD, BBa_K404112) and an improved guanylate kinase - thymidine kinase fusion gene (mGMK_TK, BBa_K404113) within the Virus Construction Kit as effective suicide genes. We demonstrate efficient and specific killing of tumor cells by enzymatic cytotoxicity assays, flow cytometry, as well as phase contrast microscopy. HT1080 cancer cell lines were transduced with directed viral particles containing the suicide genes packaged into the viral capsids.
Successful Assembly of Vector Plasmids Carrying Suicide Genes via Cloning
To create the functional vector plasmids, assembly of the constructs carrying the suicide genes was performed following the BioBrick Standard Assembly. All plasmids contain the enhancer-element human beta-globin intron ( BBa_K404107) and the human growth hormone terminator signal (hGH, BBa_K404108) flanked by the inverted terminal repeats (ITRs, BBa_K404100 and BBa_K404101). Assembled suicide genes are either under the control of the CMV promoter ( BBa_J52034) or the tumor-specific telomerase promoter phTERT ( BBa_K404106).
|
Figure 1: BioBrick-compatible assembly of functional vector plasmids containing the suicide genes. The schematic figure shows the cloning strategy of the guanylate kinase – thymidine kinase fusion gene (mGMK_TK30). |
To enable modularization of the thymidine kinase mutants TK30 and SR39 ( BBa_K404109 and BBa_K404110) according to the BioBrick standard, the fusion genes mGMK_TK30 and mGMK_SR39 ( BBa_K404113 and BBa_K404315) and CD ( BBa_K404112) were modified using the QuikChange Lightning Site-Directed Mutagenesis Kit (Stratagene) for deletion of iGEM RFC10 pre- and suffix restriction sites. Figure 2 demonstrates one example of successful deletion of a PstI restriction site located within the mGMK_TK30 sequence at position 3109. A point mutation was introduced replacing the nucleotide G by A, resulting in the deletion of the restriction site while maintaining the encoded amino acid glutamine. Exchange of guanine to adenine was confirmed by sequencing (Figure 2).
|
Figure 2: Cytosine to guanine exchange by site-directed mutagenesis using QuikChange Lightning Kit provided by Stratagene was successful as demonstrated by (A) test digestion linearizing the plasmid with PstI and (B) by sequencing. |
Furthermore, assembly of BioBrick-compatible vector plasmids was performed. An example for the last assembly step of mGMK_TK30 and hGH_rITR is shown in Figure 3. The plasmids were digested with both XbaI and PstI (New England Biolabs, Insert: BBa_K404116: hGH_rITR) or SpeI and PstI (Vector) and loaded on an agarose gel. As demonstrated in the preparative gel in Figure 3, the expected bands were detected under UV light and the extracted, subsequently purified DNA was successfully ligated and transformed into E. coli. Each assembly step for producing the BioBricks was conducted following the iGEM BioBrick standard.
|
Figure 3: Assembly of mGMK_TK30 (vector molecule, in pSB1C3) and hGH-terminator_rightITR (insert molecule). The digested fragments were visualized under UV-light and correspond to the expected sizes. |
Monitoring Efficient Tumor Killing by Phase-Contrast Microscopy
Tumor cells, transduced with viral particles encapsidating the effector constructs containing the mGMK_TK30 driven by the CMV promoter, were cultured both in presence and absence of ganciclovir (Roche). Morphological changes were monitored via phase-contrast microscopy for 48 hours post infection. As it can be seen in Figure 4, non-transduced tumor cells treated with ganciclovir and transduced cells without ganciclovir did not show significant cell ablation. In contrast, transduced cells expressing the guanylate kinase - thymidine kinase fusion protein showed significant cell death after incubation with ganciclovir for 48 hours post infection.
|
A |
B |
|
C
|
D |
|
Figure 4: Qualitative analysis of cell death induced by conversion of ganciclovir to ganciclovir-triphosphate by virus-delivered guanylate - thymidine kinase (mGMK_TK30). A: Non-transduced HT1080 cells incubated in the presence of ganciclovir not exhibiting cell death. B: Untreated transduced HT1080 cells showing a high survival rate. C: HT1080 cells transduced with 300 µL viral particles and incubated with ganciclovir leading to tumor cell ablation. D: HT1080 cells transduced with 600 µL viral particles and incubated with ganciclovir leading to ablation of tumor cells. |
|
Suicide gene therapy is based on the localized conversion of non-toxic prodrugs to toxic substances (Greco & Dachs 2001), promoting cell death in the tumor tissue (Figure 5). Directed gene delivery is achieved by using recombinant viral vectors as provided by the iGEM team Freiburg_Bioware 2010 within the Virus Construction Kit.
|
Figure 5: Overview of the suicide gene therapy approach. Non-toxic prodrugs are converted into toxic effector molecules leading to cell death of the tumor cells. |
Non-transduced cells can survive in the presence of ganciclovir since the prodrug is not toxic for these cells (Figure 4A). The demonstration that transduced cells are viable in the absence of ganciclovir confirms that cell killing is indeed induced by combination of the delivered thymidine kinase and treatment with ganciclovir. Viral particles encapsidating the suicide construct mGMK_TK30 are efficient in directed gene delivery, thus leading to cell death of transduced cells due to overexpression of mGMK_TK30 and prodrug conversion. The cell-toxic ganciclovir-triphosphate is incorporated into the nascent DNA chain leading to replication termination and finally resulting in death of dividing cells.
Quantitative Analysis of Cell Death by Flow Cytometry
Quantitative analysis of the cytotoxic effect induced by mGMK_TK30 was first conducted by flow cytometry analysis 72 hours post transduction. HT1080 cells were stained with 7-AAD and Annexin V. 7-AAD intercalates in double-stranded DNA after penetrating cell membranes of dead cells, whereas Annexin V specifically binds phosphatidylserine which is only accessible during apoptosis. Figure 6 demonstrates the relation between cell death and ganciclovir concentration.
|
|
|
|
Figure 6: Flow cytometry data analysis. A: Gating non-transduced HT1080 cells (control). B: Non-transduced cells without staining plotted against 7-AAD. C: Gating non-transduced cells stained with 7-AAD. D: Non-transduced, 7-AAD-stained cells plotted against 7-AAD. E: Gating transduced cells (GOI: mGMK_TK30) treated with 485 µM ganciclovir. F: Gated, Annexin-V stained cells plotted against AnnV-2 Log. G: Gated cells plotted against 7-AAD H: Gated, 7-AAD and Annexin-V stained cells plotted against 7-AAD and Annexin-V. Gate R19 comprised Annexin-V and 7-AAD positive cells. |
|
|
Figure 7: Quantification of flow cytometry data provided in Figure 6. With increasing ganciclovir concentration, the survival rate of cells decreases. 60 % of HT1080 cells treated with 4.85 mM ganciclovir show tumor ablation, however even lower amounts of ganciclovir lead to significant cell death. |
The effect of different ganciclovir concentrations on transduced HT1080 sarcoma cells was investigated. Transduction was performed with recombinant viral particles encapsidating the mGMK_TK30 prodrug gene. 72 hours post infection, cells were stained with 7-AAD and Annexin V. As Figure 7 shows, the fraction of killed transduced cells is proportional to the applied ganciclovir concentration.
Titrating Ganciclovir Concentrations for Efficient Cell Killing by Cytotoxicity Assays
Further analysis of the cytotoxic effect induced by thymidine kinase converting ganciclovir to the toxic anti-metabolite has been performed using MTT assays. 3-(4,5-Dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide), also known as MTT, is a yellow-colored tetrazole, which is reduced to purple insoluble formazan in the presence of NADH and NADPH (Roche n.d.). Colorimetric analysis can be carried out via spectrometry. Different tumor cell lines, HT1080 and A431, were transduced with the recombinant viruses carrying the linear DNA construct coding for mGMK-TK30 regulated by the CMV promoter and subsequently treated with ganciclovir. 48 and 72 hours post infection, cells were incubated with MTT and fresh DMEM. After cell lysis by DMSO, absorbance of formazan at 570 nm was quantified using a Tecan Sunrise plate reader.
|
A |
|
B
Figure 8: Effect of ganciclovir on HT1080 cell survival 72 hours post infection as (A) two dimensional plot of survival of cells and (B) three-dimensional plot of ganciclovir, virus particles and cell survival. |
|
A
|
|
B
Figure 9: Effect of ganciclovir on HT1080 cell survival 96 hours post infection. (A) two dimensional plot of cell survival (B) three-dimensional plot of ganciclovir, virus particles and cell survival. |
Data of MTT assay quantification are shown in Figure 8 and Figure 9. HT1080 cells were infected with viral particles containing the mGMK_TK30 transgene. 72 h- and 96 h post infection and addition of ganciclovir, cells were incubated with MTT. Changes in absorbance were measured and survival of cells plotted against ganciclovir concentration. Figure 8A demonstrates the correlation between increasing ganciclovir concentrations and percentage of cell survival. Furthermore, different virus particle concentrations were used for transduction. Figure 8B shows that the highest amount of viral particles combined with the highest ganciclovir concentration led to significant HT1080 apoptosis 72 hours post transduction.
Additionally, 96 hours post infection cells were incubated with MTT and absorbance was quantified via spectrometry (Figure 9). Again, survival of HT1080 cells was plotted against increasing ganciclovir concentrations.
Conclusions
Efficient and tissue-specific tumor killing is one major challenge in cancer therapy (Black et al. 1996). Gene-directed enzyme prodrug therapy (GDEPT) is based on the conversion of non-toxic substances into toxic drugs resulting in tumor cell death. The iGEM team Freiburg_Bioware 2010 provides several functional suicide genes within the Virus Construction Kit, thus offering a feasible and modular tool to the growing field of personalized medicine and the iGEM community. We successfully demonstrated cancer cell death caused by the introduction of modified fusion genes consisting of guanylate and thymidine kinases.
To prevent systemic toxic side effects of conventional chemotherapy, the iGEM team Freiburg_Bioware 2010 took a leap and efficiently retargeted the viral vector for directed suicide gene delivery towards tumor cells. Capsid engineering was successfully demonstrated by the iGEM team Freiburg_Bioware 2010. Further details can be found under iGEM wiki Freiburg_Bioware 2010 - Results - Targeting
Individual components of the assembled vector plasmid
ITRs

Figure 10: ITR details
The inverted terminal repeat structures can be subdivided into several palindromic motives: A and A’ form a stem loop which encases B and B’ as well as C and C’. Those motives form both arms of the T-shaped structure. The functional motives on the ITR are two regions that bind Rep 68/78, called Rep-binding elements (RBE on the stem and RBE’ on the B arm) and the terminal resolution site (trs) in which the rep proteins introduce single-stranded nicks. The 3’ OH end of the A motive acts as a primer for DNA replication (Im & Muzyczka, 1990) (Lusby, Fife, & Berns, 1980).
Beta-globin-intron

Figure 11 A: FACS data

Figure 11 B: Calculated percentage of mVenus expressing cells
The beta-globin intron BioBrick consists of a partial chimeric CMV promoter followed by the intron II of the beta-globin gene. The 3´end of the intron is fused to the first 25 bases of human beta globin gene exon 3. The beta globin intron BioBrick is assumed to enhance eukaryotic gene expression (Nott, Meislin, & Moore, 2003). As shown in Figure 11 A and B the vectorplasmid missing the beta-globin intron showed a negligible difference in mVenus expression compared to viral genomes containing the beta-globin intron. Considering these results and taking into account that a constant volume of viral particles has been used for transduction, the difference between the construct containing and lacking the beta-globin intron is minimal. Since packaging efficiency of the AAV-2 decreases with increasing sizes of the insert (Dong, Fan, & Frizzell, 1996), the iGEM team Freiburg_Bioware suggests using the beta-globin intron in dependence on the size of your transgene.
mGMK_TK30 fusion protein
The thymidine kinase mutant TK30 contains six modified amino acids (Black, Newcomb, Wilson, & Loeb, 1996) created in a first screening showing enhanced affinity for gancivlocir and acyclovir, but reduced specificity for its natural substrate thymidine.
As efficient tumor killing and therefore ganciclovir activation is essential for successful tumor ablation, further improvements were conducted. Overexpression of transgenic thymidine kinase leads to accumulation of non-toxic intermediates, which cannot be phosphorylated sufficiently by endogenous guanylate kinase, the second enzyme in the salvage pathway of nucleotides.
Overcoming this bottleneck was accomplished by fusing the mouse guanylate kinase (mGMK) to the N-terminus of TK30 mutant creating a fusion protein (mGMK_TK30) with enhanced GCV/ACV sensitivity in vitro and in vivo (Ardiani, Sanchez-Bonilla, & Black, 2010) and improved bystander activity. The effect of non-transfected tumor cell killing upon transfer of toxic metabolites through gap junctional intercellular communication (GJIC) or immune-mediated tumor ablation is essential in suicide gene therapy (Pope, 1997). GCV-triphosphate is mainly transported through the central pore formed between connexin proteins from neighboring cells (Gentry, Im, Boucher, Ruch, & Shewach, 2005), but immune-induced bystander effect seems to be likely as well (Grignet-Debrus, Cool, Baudson, Velu, & Calberg-Bacq, 2000).
hGH termination signal
Recombinant AAV genomes were engineered containing the inverted terminal repeats (ITRs), a strong eukaryotic promoter and mVenus as gene of interest with and without the hGH terminator signal. Transduction of HT1080 cells with viral particles containing the rAAV genomes and measuring mVenus expression 24-hours post infection by flow cytometry demonstrated that transgene expression of the constructs lacking the hGH termination signal is significantly reduced. The iGEM team Freiburg_Bioware 2010 therefore suggests using the provided hGH termination signal within the Virus Construction Kit for optimal gene expression.
Since cloning does not confirm biological activity, we analyzed the plasmids and their functional components, hGH terminator and beta-globin intron, in cell culture. Assembled plasmids have been cotransfected, using AAV-293 cells, which provide the stable integrated E1A and E1B genes, with helper plasmids required for capsid assembly and genome encapsidation (pRC and pHelper) in a molar ratio of 1:1:1 (pGOI:pRC:pHelper). Virus particles containing the single stranded DNA were harvested 72-hours post transfection and HT1080 cells transduced with constant volumes of viral vectors. 48-hours post infection; transduced cells expressing the gene of interest were analyzed by flow cytometry.
Recombinant vectorplasmids were engineered containing the inverted terminal repeats (ITRs), a strong eukaryotic promoter (CMV promoter: BBa_K404102) and mVenus as gene of interest with and without the hGH terminator signal. Transduction of HT1080 cells with constant volume of viral particles containing the vectorplasmids and measuring mVenus expression 24-hours post infection by flow cytometry demonstrated that transgene expression of the constructs lacking the hGH termination signal is significantly reduced as shown in confirming the expected results that hGH is essential for mRNA processing. The iGEM team Freiburg_Bioware 2010 therefore suggests using the provided hGH termination signal within the Virus Construction Kit for optimal gene expression.
 |
 |
| Figure 12: Flow cytometry analysis of vectorplasmid without (left) and with (right) hGH. For viral particle production, AAV-293 cells were transfected with the reassembled vector plasmid (BBa_K404119) or the reference plasmid, respectively. A: Gating non transduced cells (control); subcellular debris and cellular aggreates can be distinguished from single cells by size, estimated forward scatter (FS Lin) and granularity, estimated side scatter (SS Lin) B: Non-transduced cells plotted against cells expressing mVenus (Analytical gate was set such that 1% or fewer of negative control cells fell within the positive region (R5) C: Gating transduced cells (R2 ≙ R14) (plasmids used for transfection: pGOI: pSB1C3_lITR_CMV_beta-globin_mVenus_hGH_rITR (pSB1C3_mVenus: BBa_K404119), pHelper, pRC. D: Transduced cells plotted against cells expressing mVenus. R10 comprises transduced cells detected by mVenus fluorescence. E: Overlay of non-transduced (red) and transduced (green). |
F: Gating non-transduced cells (control). G: Non-transduced cells plotted against cells expressing mVenus (R5). H: Gating transduced cells (R14 ≙ R2) (plasmids used for transfection: pGOI: pAAV_mVenus, pHelper). I: Transduced cells plotted against cells expressing mVenus. R10 comprises transduced cells detected by mVenus fluorescence. J: Overlay of non-transduced (red) and transduced (green) cells plotted against mVenus expression. |

Figure 13: Calculated percentage of mVenus expressing cells
Flow cytometry analysis of vectorplasmids with and without hGH terminator. YFP expression of viral genomes was determined by flow cytomery after 24-hour post infection. Results demonstrate that mVenus expression of vectorplasmids lacking the hGH terminator is reduced significantly proving that the polyadenylation signal is essential for viral gene expression using recombinant viral vectors engineered by using components of the Virus Construction Kit.
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1319
Illegal NgoMIV site found at 1977
Illegal NgoMIV site found at 2882
Illegal AgeI site found at 3011 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 3413
Illegal SapI.rc site found at 1369
References
Ardiani, A., Sanchez-Bonilla, M. & Black, M.E., 2010. Fusion enzymes containing HSV-1 thymidine kinase mutants and guanylate kinase enhance prodrug sensitivity in vitro and in vivo. Cancer gene therapy, 17(2), 86-96. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2808426&tool=pmcentrez&rendertype=abstract.
Black, M.E. et al., 1996. Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy. Proceedings of the National Academy of Sciences of the United States of America, 93(8), 3525-9. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=39643&tool=pmcentrez&rendertype=abstract.
Fuchita, M. et al., 2009. Bacterial cytosine deaminase mutants created by molecular engineering show improved 5-fluorocytosine-mediated cell killing in vitro and in vivo. Cancer research, 69(11), 4791-9. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2765227&tool=pmcentrez&rendertype=abstract.
Fuchita, M. et al., 2009. Bacterial cytosine deaminase mutants created by molecular engineering show improved 5-fluorocytosine-mediated cell killing in vitro and in vivo. Cancer research, 69(11), 4791-9. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2765227&tool=pmcentrez&rendertype=abstract.
Greco, O. & Dachs, G.U., 2001. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. Journal of cellular physiology, 187(1), 22-36. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11241346.
Huber, B.E. et al., 1993. In vivo antitumor activity of 5-fluorocytosine on human colorectal carcinoma cells genetically modified to express cytosine deaminase. Cancer research, 53(19), 4619-26. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8402637.
Moolten, F.L., 1986. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer research, 46(10), 5276-81. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3019523.
Roche, Apoptosis , Cell Death and Cell Proliferation,
Willmon, C.L., Krabbenhoft, E. & Black, M.E., 2006. A guanylate kinase/HSV-1 thymidine kinase fusion protein enhances prodrug-mediated cell killing. Gene therapy, 13(17), 1309-12. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16810197.